-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
The spatially non-overlapping function of Hox genes is known to determine Antero-posterior body axis in all the bilaterians. The expression of Hox genes is found to be overlapping in several cases. According to the posterior prevalence rule, posterior Hox genes suppress the function of anterior Hox genes in the overlapping expression domains. Our findings show an exception to the rule of posterior prevalence. We show that in the overlapping expression domains of abd-A and Abd-B in early pupal abdominal epithelia, both the genes have essential roles. While abd-A is required for cell proliferation, Abd-B determines the segmental identity.
Published in the journal: Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule. PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004717
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004717Summary
The spatially non-overlapping function of Hox genes is known to determine Antero-posterior body axis in all the bilaterians. The expression of Hox genes is found to be overlapping in several cases. According to the posterior prevalence rule, posterior Hox genes suppress the function of anterior Hox genes in the overlapping expression domains. Our findings show an exception to the rule of posterior prevalence. We show that in the overlapping expression domains of abd-A and Abd-B in early pupal abdominal epithelia, both the genes have essential roles. While abd-A is required for cell proliferation, Abd-B determines the segmental identity.
Introduction
Anteroposterior (AP) body axis in all the bilaterians is determined by a set of homeobox (Hox) containing genes, the Hox genes [1]. The eight Hox genes in Drosophila are arranged in two clusters, the Antennapedia complex (ANT-C) and the bithorax complex (BX-C) [2]–[4]. BX-C has three Hox genes Ultrabithorax (Ubx), abdominal-A (abd-A) and Abdominal-B (Abd-B) [3], [5]. During development, Hox genes express in a collinear manner where the order of genes in the genomic locus is similar to their spatial expression pattern along the AP axis of the embryo [6]–[9]. This expression pattern of Hox genes determines the identity of the body segments along the AP body axis [10], [11]. In several instances, expression of Hox genes is found to be overlapping [9]. In such cases, posteriorly expressed Hox genes are known to suppress function of anterior genes at transcriptional or post-translational level of gene regulation, a phenomenon known as posterior dominance [10], [12]–[15]. Therefore, the function of the anterior Hox gene in such regions of overlapping expression with the posterior Hox gene is thought to be irrelevant. This is supported by the observation that mutants for anterior Hox genes do not show distinct phenotypes in the region of overlapping expression up to early larval stage and die later during development. Owing to this, the role of anterior Hox genes in the region where they co-express with posterior ones has not been investigated extensively during larval and pupal stages. A few studies, however, suggest that anterior Hox genes can have non-homeotic functions in the region of co-expression with posterior Hox genes [16], [17].
In order to investigate the role of three Hox genes Ubx, abd-A and Abd-B in their non-overlapping and overlapping domains of expression, we analyzed their expression pattern in the early pupal abdominal epithelial cells. Each abdominal segment at larval stage has two cell types the polytenized larval epithelial cells (LECs) and diploid histoblast nest cells (HNCs). The HNCs are maintained in a quiescent state during the larval development and proliferate during the early pupal development to differentiate into adult abdominal epithelial cells [18], [19]. During this process, HNCs induce apoptosis in the LECs and replace them with a layer of abdominal epithelium. For consistency we have used early pupal abdominal epithelia of 0 to 32 h after puparium formation (APF) having both LECs and HNCs. The abdominal epithelia formed after 32 h APF by proliferation and differentiation of HNCs and removal of LECs are termed as pupal abdominal epithelia. These pupal abdominal epithelial cells further develop into adult abdominal epithelia with features like bristles and pigmentation. We found that Ubx is expressed only in HNCs and LECs of first segment of early pupal abdominal epithelia and does not overlap with abd-A or Abd-B. On the contrary, abd-A co-expresses with Abd-B in HNCs and LECs. Here we show that in the coexpressing HNCs, abd-A is required for formation of adult abdominal epithelia while Abd-B is required for its identity. We also observed that the higher expression of Abd-B in abdominal segment 7 suppresses expression of abd-A that leads to smaller segment in females and complete elimination in males. These findings, for the first time, show that abd-A is required for abdominal epithelia formation not only in its exclusive expression domain but also in the region where it overlaps with Abd-B.
Results
Expression analysis of Ubx, abd-A and Abd-B in early pupal abdominal epithelia
Three Hox genes of the BX-C in Drosophila melanogaster determine the identity of third thoracic and all abdominal segments [9], [20]. The identity of third thoracic (T3) and first abdominal segment (A1) is determined by Ubx, A2 to A4 by abd-A and A5 to A9 by Abd-B [9]. Although these Hox genes regulate the identity of specific segments in adults, their expression is not restricted only to the corresponding parasegments (PSs) in embryos [7], [21], [22]. The Ubx gene is expressed from PS5 to PS12, abd-A expresses from PS7 to PS12 while Abd-B from PS10 to PS14 in embryos [6], [7], [23]. This results in the overlap of Ubx with abd-A in PS7 to PS9, and all three Hox genes in PS10 to PS12 (Figure 1A, Figure S1A and B) [8], [21]. This overlapping expression is seen not only in the same parasegment but also in the same cells (Figure S1A and B), raising the question of why an anterior Hox gene should express in the domain of a posterior Hox gene if it has no apparent functions there.
Fig. 1. Immunostaining of Ubx, abd-A and Abd-B. 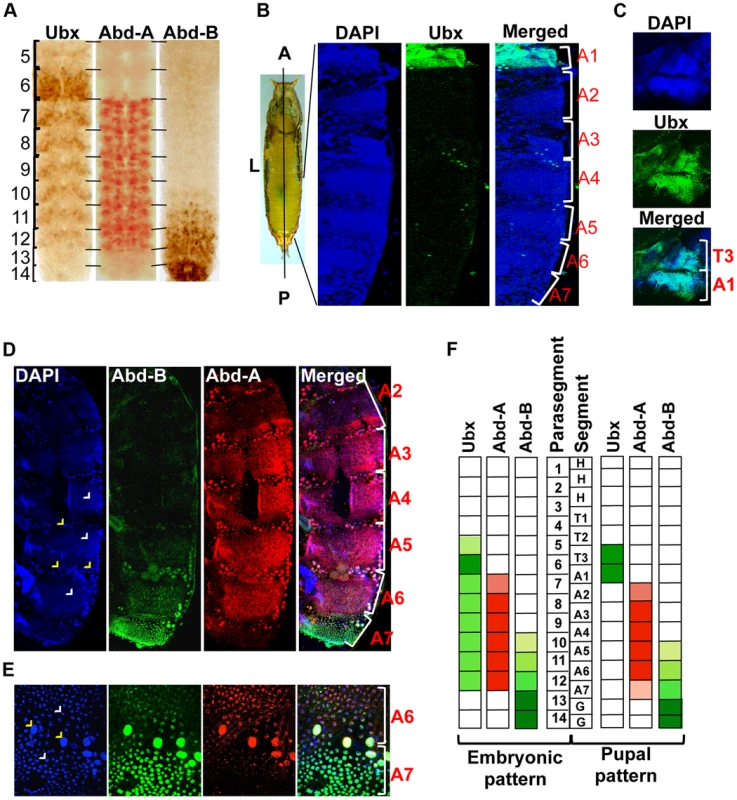
A) Expression pattern of three BX-C genes are shown in parasegments (PS) of embryonic CNS, placed anterior towards up. B) Whole pupae were cut longitudinally from dorsal side to isolate two halves of early pupal abdominal epithelia. The expression pattern of Ubx is seen only in LECs and HNCs of A1 and not in posterior segments. The early pupal abdominal epithelium is placed with anterior at the top. C) The expression of Ubx is also seen in T3 apart from A1 segment. D) Expression of Abd-A is seen in LECs (yellow arrowheads) and HNCs (white arrowheads) of A2–7, although the expression in A2 and A7 is lower in comparison of other segments. Expression pattern of Abd-B shows a gradient from A5 to A7, where A5 shows minimum and A7 shows maximum expression. E) Magnified picture of A6 and A7 segment shows the expression of Abd-A and Abd-B in LECs (yellow arrowheads) and HNCs (white arrowheads) of A6 and A7. The expression of Abd-A is weaker in A7 in comparison to that of in A6. F) A pictorial representation of expression pattern of the three Hox genes in embryonic PS compared with their expression in the corresponding segments in early pupal abdominal epithelia. We further explored if the overlapping expression pattern seen during embryonic development also persists in LECs and HNCs of early pupal abdominal epithelia. In immunostaining experiments, we observed the expression of Ubx in T3 and A1 segment, Abd-A from A2–A7 and Abd-B from A5–A7 (Figure 1B, C and D respectively), which is similar to earlier observations [24], [25]. The expression of all the three genes is seen in bigger polytenised LECs (yellow arrowheads) and smaller diploid HNCs (white arrowheads). In contrast to the embryonic expression pattern, Ubx is observed only in third thoracic (T3) and first abdominal segment (A1) and not in any of the posterior segments (Figure 1B and C). This suggests that Ubx expression does not overlap with the other two genes while overlapping expression of Abd-A and Abd-B is seen from A5 to A7 (Figure 1D). A closer look reveals that the expression of abd-A is not uniform and shows very weak expression in A2 and A7 in comparison to other segments (Figure 1D). The expression of Abd-B continues to show a lower to higher gradient from A5 to A7 as seen in embryonic CNS (Figure 1A and D). Furthermore, we observed that from A5 to A7 the Abd-A and Abd-B expression coexists not only in same segment but also in the same nuclei (Figure 1E). These observations establish that, unlike the embryonic expression pattern, at pupal stage the expression of Ubx does not overlap with abd-A or Abd-B while the overlap between abd-A and Abd-B persists in LECs and HNCs of early pupal abdominal epithelia (Figure 1F).
Functional analysis of abd-A in adult abdominal epithelia development
To assess the role of abd-A and Abd-B in both overlapping and non-overlapping expression domains, we chose to knock down abd-A and Abd-B in HNCs and LECs using UAS-RNAi and Gal4 approach. We used esg-Gal4 and Eip-71CD-Gal4 for exclusive knockdown in HNCs and LECs, respectively and 71B-Gal4 and Pnr-Gal4 for knockdown in the both cell types [26], [27] (Figure 2 A1-4). The esg-Gal4 is known to express in all histoblast nest cells in early pupa (before 24 h), which fades away later in development (Figure 2 A1) [19]. We found that the expression of 71B-Gal4 is seen mainly in larval epithelial cells till 18h APF but later it also starts expressing in HNCs at low levels and increases with time. Interestingly, its expression in HNCs is stochastic and not uniform as seen in esg-Gal4. (Figure 2 A3 and Figure S2). The expression of Pnr-Gal4 is not seen in early pupa and starts expressing in LECs after 14 h APF, which later increases and is seen in all LECs and in few rows of HNCs that are leading towards dorsal midline (Figure 2 A4 and Figure S3).
Fig. 2. abd-A is required for abdominal epithelia development. 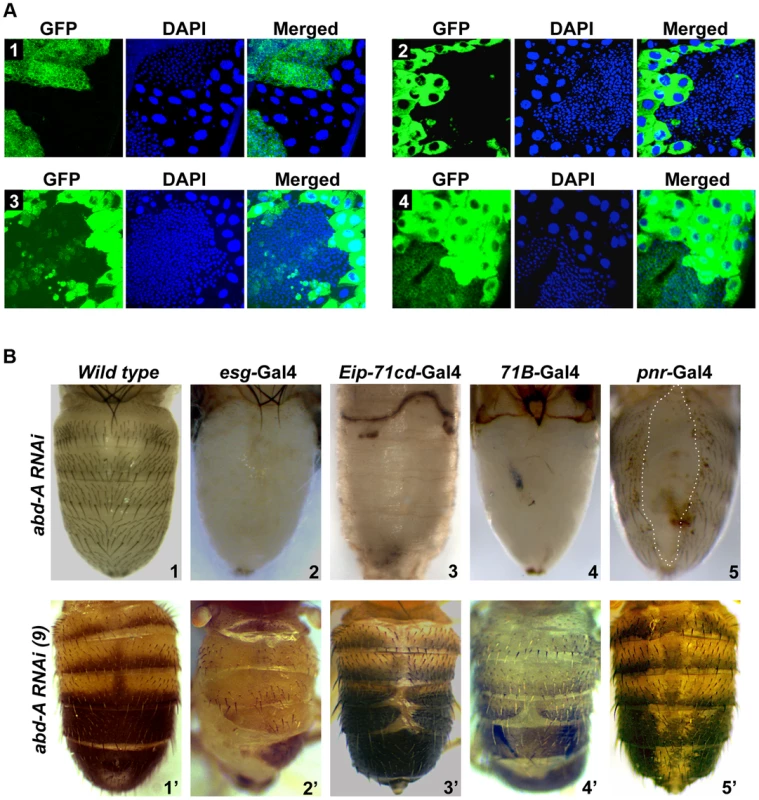
A1–4) Expression of esg, Eip-71CD, 71B and Pnr Gal4 lines, respectively, are shown using UAS-GFP. The expression of esg-Gal4 is seen in HNCs of 22 h APF, Eip-71CD-Gal4 only in LECs and 71B and Pnr Gal4s in both LECs and HNCs of 26 h APF. B1-5) Abdominal segments of wild type (B1) and abd-A RNAi pharates using various Gal4s are shown as indicated. B1′–5′) Phenotype of abdominal tergites in wild type and weaker abd-A RNAi line using various Gal4 lines, as indicated on the top of the panel. Abdominal part of pharates and adult flies are placed facing dorsal side and anterior at the top. Knockdown of abd-A in HNCs using esg-Gal4 driver shows lethality at larval stages and only ∼20% larvae pupated although none of them hatch. These pupae showed complete loss of abdominal epithelia in the expression domain of abd-A (A2–A7) but not in A1 (Figure 2 B2) suggesting this to be an abd-A specific phenotype. The loss of epithelia was observed from both dorsal and ventral sides of the segment and no tergite or sternite was seen in these segments (Figure 2 B2 and Figure S4). This brings out the critical role of abd-A in HNCs for development of adult abdominal epithelia at pupal stage of development. The knockdown of abd-A in LECs using Eip-71CD-Gal4 shows complete lethality at very early stage (before 24 h) of pupal development. These pupae did not even grow enough to show any epithelia formation (Figure 2 B3). The simultaneous knocking down of abd-A in both LECs and HNCs using 71B-Gal4 also shows developmental arrest at pupal stage leading to lethality. Manually eclosed pharates show loss of abdominal epithelia similar to what we see with esg-Gal4 (Figure 2 B4). Similarly, the abd-A knockdown using Pnr-Gal4 also showed lethality at pupal stage but manually eclosed pupae showed dorsal closure defect of abdominal epithelia (DDA) (Figure 2 B5: marked by dotted line). In this case we observed loss of epithelia only close to dorsal mid line, which corresponds to the expression pattern of this Gal4 driver in LECs and HNCs.
We also generated milder abd-A RNAi lines (see materials and methods) and used them to knockdown abd-A in HNCs and LECs. As expected, these lines gave less severity and penetrance of the phenotypes. The phenotype of one of the lines is shown in figure 2 B2′-5′. Knockdown of abd-A in HNCs using this line with esg-Gal4 shows less lethality at pupal stage and adult flies show partial loss of abdominal epithelia in adults, which is always restricted to A2–6 (Figure 2 B2′). The adult abdominal epithelium of the knockdown flies also show lesser and smaller bristles in comparison to the wild type flies (Figure 2 B2′ and 2B1′ respectively). This indicates the homeotic transformation of the epithelia of posterior segments into A1 segment that has smaller bristles. This surprised us because in the abd-A knockdown the features of posterior segments transform into features similar to Ubx expression domain A1, although we did not observe Ubx expression in HNCs of these segments. We reasoned that the knockdown of abd-A might be leading to the derepression of Ubx in posterior segments thus showing this phenotype. To test this, we did immunostaining of Ubx in abd-A RNAi background. We observed the expression of Ubx only in HNCs of posterior segments (Figure S5), which was not seen in wild type epithelia (Figure 1B), suggesting that in wild type epithelia abd-A suppresses expression of Ubx in its expression domain.
Furthermore, knockdown of abd-A using Eip-71CD-Gal4 and 71B-Gal4 with milder abd-A RNAi line also showed viability and DDA phenotypes in adults (Figure 2 B3′–4′). The DDA phenotype is always restricted to A2 to A6 segment. Knockdown of abd-A by Pnr-Gal4 shows loss of pigmentation across the dorsal midline, suggesting that the expression of abd-A is also required for pigmentation of adult cuticle (Figure 2B5′). The phenotype of these abd-A RNAi experiments shows that the knockdown of abd-A in HNCs leads to a loss of epithelial cells while that in LECs causes DDA phenotype. This indicates that the level of abd-A is critical in HNCs and LECs during development of adult abdominal epithelia at pupal stage. Interestingly this role of abd-A in epithelia formation is not limited only in its exclusive expression domain but also in the domain where it overlaps with Abd-B (A5 and A6 segments).
Role of abd-A expression in the HNCs
We further assessed the role of abd-A in HNCs using live cell imaging. The HNCs are quiescent during larval stage and get signal to proliferate as soon as pupation starts. The proliferation of HNCs is biphasic, where in the first phase (4–12 hours APF), cells divide without growing much in size while in the second phase (15–36 hours APF) cells divide normally and make complete epithelia [19]. In the abd-A knockdown using esg-Gal4 the live cell imaging of HNCs showed almost 50% reduction in the proliferation of HNCs during first six hours of the first phase of proliferation (Figure 3A). There are 16 HNCs at 0 h APF, after 6 h of proliferation their number become 60±5 HNCs in the case of esg-Gal4 while it is only 31±5 in the case of abd-A RNAi. We also observed that about 30% of the analyzed HNCs show complete arrest at this stage and do not proliferate. Further analysis of LECs and HNCs in these pupae also showed that non-proliferating HNCs are unable to eliminate LECs, which results in the arrest in development of abdominal epithelia (Figure 3B and C). This clearly shows that abd-A is required in HNCs for their proliferation during early pupal development.
Fig. 3. abd-A is required in HNCs for their proliferation. 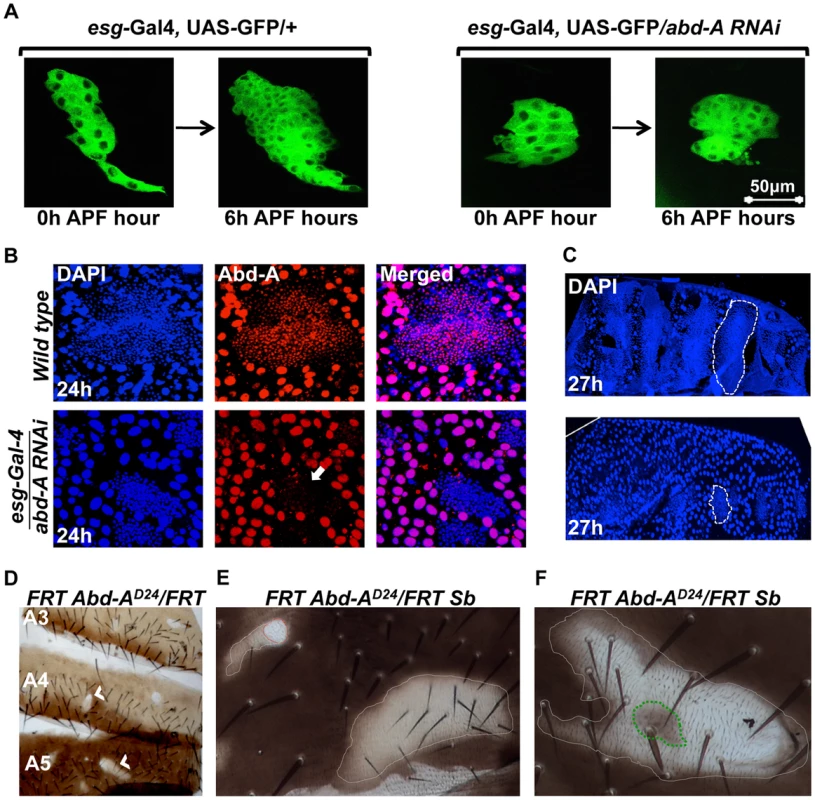
A) Live cell imaging using esg-Gal4 UAS-GFP shows reduced proliferation of HNCs in the case of abd-A RNAi. B) Immunostaining of 24 h old pupal abdominal epithelial cells in wild type and in the abd-A RNAi with esg-Gal4 show cell type specific loss of abd-A and less number of HNCs in the case of abd-A RNAi (white arrow) in comparison to wild type. The DAPI stained nuclei of LECs (bigger in size) in wild type epithelia are not spherical and show irregular shape indicating HNC induced cell death while in the case of abd-A RNAi these nuclei show round shape. C) Lower panel of 27 h APF abdominal epithelia (placed anterior left) in abd-A RNAi using esg-Gal4 show less number of HNCs (marked by dotted lines) and more number of LECs in comparison to wild type (upper panel). D) Mitotic clones of abd-A mutant are seen in all three abdominal segments. E) The abd-A clones white in color are small without bristles (red dotted lines) while mitotic clones with lighter pigmentation show small bristles (white dotted lines). F) This clone of abd-AD24 is made using stubble (Sb) mutation (gives stubble bristle) on other homologous chromosome. We see a stubble bristle in middle of the clone while normal bristles are mainly present in the light pigmented areas and white patch show rare or no bristles. To further analyze the role of abd-A in the HNCs during the development of adult epithelia, we made mitotic clones of abd-A using its loss of function allele, abd-AD24 [28]. Mitotic clones were observed in all segments from A2 to A6 implying a common role of abd-A in all these segments (Figure 3D). Mitotic clones are seen as either small white patches on the tergites without bristles (Figure 3E, red dotted line) or relatively larger light pigmented regions with small bristles (Figure 3E, white dotted line). One of such white clones shows bristles only at the places where pigmentation is low while white areas are devoid of bristles (Figure 3F). In the middle of the clone we also see a pigmented patch (marked with green dotted line) with stubble bristle representing an abd-A positive region. The small bristles in the mitotic clones suggest that these cells are transformed into A1 like cells as seen on knocking down of abd-A in HNCs (Figure 2B2′). We further extended our analysis and gave heat shock at 0 h, 12 h, 24 h and 36 h APF to evaluate the role of abd-A in HNCs during pupal development. We observed that mitotic clones show phenotypes only in the flies that were given heat shock at 0 h, 12 h APF and not later stages. All pupae (40 out of 40) that were given heat shock at 0 h APF show phenotype in mitotic clones of adult flies while only 15% of (6 out of 40) pupae that were given heat shock at 12 h APF show phenotype in mitotic clones. These results confirmed the abd-A RNAi result that abd-A is required in HNCs for identity and proliferation during the first phase of HNC proliferation. These observations also suggest that expression of abd-A is required for normal size bristles not only in its exclusive expression domain but also in the expression domains (A5 and A6) where it overlaps with Abd-B.
Role of abd-A expression in the LECs
We investigated the role of abd-A in LECs by knocking down abd-A using Eip-71CD- Gal4. Since the original strong abd-A RNAi line gave early pupal lethality, we used a milder RNAi line. In this case also, we observed LEC specific reduction in abd-A expression (Figure 4A). These LEC nuclei do not show the distinct apoptotic feature of irregular nuclear morphology, unlike what is seen in wild type nuclei (Figure 4A). The development of these pupae was arrested during early pupal stage and we see that HNCs do not grow and LECs are not removed as compared to wild type (Figure 4A and B). This suggests that the loss of abd-A prevents cell death in LECs and, thereby, leaves no space for HNCs to proliferate and make complete abdominal epithelia. To confirm if the persistence of LECs during early pupal development leads to DDA phenotype as seen in abd-A knockdown we over-expressed anti-apoptotic factor P35 in LECs using Eip-71CD Gal4. These flies show DDA phenotype in all the abdominal segments (Figure 4C) similar to what is seen in abd-A RNAi using Eip-71CD- Gal4 (Figure 2B3′). This demonstrates that inefficient clearing of LECs prevents HNCs from growing and leads to fusion of abdominal epithelia at dorsal midline resulting in DDA phenotype.
Fig. 4. Expression of abd-A is required in LECs for their removal. 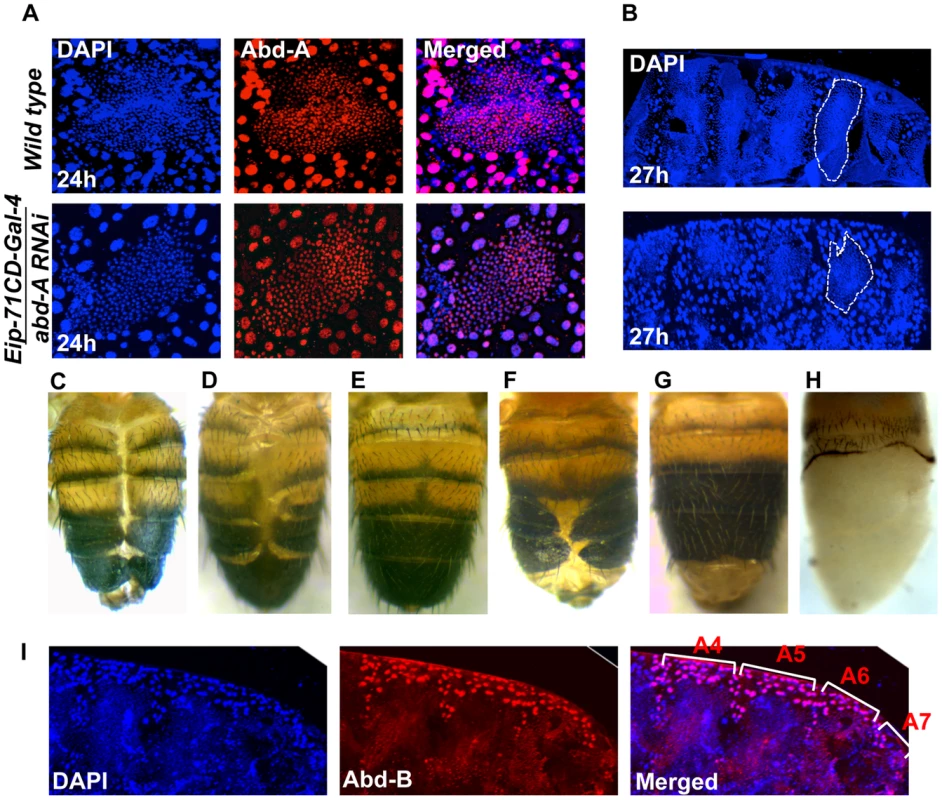
A) abd-A RNAi using Eip-71CD-Gal4 show LECs specific reduction of Abd-A level (lower panel) in comparison to wild type (upper panel). Loss of abd-A from LECs shows spherical morphology of nuclei suggesting that the cell death is not induced in these cells. B) The abd-A RNAi (lower panel) in LECs show more number of LEC nuclei and less number of HNC nuclei as compared to the wild type 27 h APF epithelia. C) Over expression of antiapoptotic protein P35 in LECs using Eip-71CD-Gal4 show DDA phenotype. D) Knockdown of abd-A in LECs by over expressing miR-iab-8-5p using Eip-71CD-Gal4 shows DDA phenotype while over expression of miR-iab-4-5p does not show DDA phenotype (E). F) A cis-regulatory mutant McpH27 Fab-71 also shows segment specific DDA phenotype. G) We observed that the DDA phenotype of McpH27 Fab-71 is rescued on knocking down Abd-B using Eip-71CD-Gal4. H) Ectopic expression of Abd-B in LECs using Eip-71CD-Gal4 shows complete loss of abdominal epithelia from A2 to A7 segment of pharate. I) Immunostaining of abdominal epithelia at 26 h (APF) shows higher expression of Abd-B (red) in LECs (bigger nuclei-stained with DAPI-blue in color) from A4 to A6 in comparison to wild type (Figure 1C). The early pupal abdominal epithelium is placed posterior at right and lateral on the top. Finally, to validate the abd-A RNAi results we also carried out loss of abd-A function by other independent ways. As miR-iab-8-5p is known to knock down Antp, Ubx and abd-A [29], [30], we overexpressed this miRNA using Eip-71CD-Gal4. Here we observed pupal lethality with few flies emerging with DDA phenotype, similar to what is seen in corresponding abd-A RNAi flies (Figure 4D and 2 B3′, respectively). In the control experiment with miR-iab-4-5p over expression, which knocks down only Antp and Ubx but not abd-A, this phenotype is not observed indicating that DDA phenotype seen in miR-iab-8-5p overexpression is specifically due to abd-A knockdown (Figure 4E). Similarly, we also observed the DDA phenotype in a cis-regulatory mutant, McpH27 Fab71, with 100% penetrance (Figure 4F). This mutant has deletion of boundary and PRE combination from Mcp and Fab7 regions, which regulates Abd-B gene in segment specific manner [31], [32]. We hypothesize that deletion of these cis-regulatory elements leads to ectopic expression of Abd-B in LECs of anterior segment causing suppression of the anterior gene abd-A that results into DDA phenotype. Immunostaining for Abd-B protein in McpH27 Fab-71 mutant indeed shows ectopic and enhanced expression of Abd-B in LECs of A4 and A5 segments (Figure 4I). Further, we knocked down Abd-B using Eip-71CD-Gal4 and observed complete rescue of the DDA phenotype in almost 55% of the flies, while rest of them showed partial rescue confirming that the ectopic expression of Abd-B causes DDA phenotype (Figure 4G). To further establish Abd-B dependent loss of abdominal epithelia, we over expressed Abd-B in LECs using Eip-71CD-Gal4 and observed 100% lethality at pupal stage. Most of the pupae died at early stages but few (15%) of them survived till later stages and showed the anticipated loss of abdominal epithelia (Figure 4H). This partial to complete loss of abdominal epithelia by Abd-B over expression is similar to what we observed in the case of abd-A RNAi (Figure 2 B2 and 4). These results confirm our abd-A knockdown results and establish that abd-A is required in LECs for their removal during abdominal epithelia development. Taken together, these observations suggest that abd-A plays dual role during development of abdominal epithelia, on the one hand it is required for proliferation of HNCs and on the other hand it is required for removal of LECs.
Functional analysis of Abd-B in abdominal epithelia development
We also did cell type specific knockdown of Abd-B to understand its role in abdominal epithelia formation. To analyze the cell type specific knockdown of Abd-B in HNCs and LECs we did immunostaining against Abd-B protein in early pupal abdominal epithelia in wild type and knockdown background. We observed HNC specific loss of Abd-B in the case of knockdown using esg-Gal4 and LEC specific loss of Abd-B with Eip-71CD-Gal4 (Figure 5A). Knockdown of Abd-B in HNCs of all the abdominal segments using esg-Gal4 driver leads to anteriorization of A5-A7 segments (Figure 5B) while adult epithelia formation is unaffected. The loss of pigmentation in A5 indicates its transformation to A4 while appearance of bristles in sternites of A6 is indicative of A6 to A5 transformation. We also see the homeotic transformation of A7 into A5 that is evident from its appearance as a discrete segment, which is otherwise absent in males [33], [34], and bristles in the sternite (Figure 5B). The transformation of these posterior segments into anterior is a typical Abd-B loss of function phenotype. We also observed defective genital and anal organs in these knockdown flies, which is in line with the known role of Abd-B in genital and anal development [35]. On the other hand, knocking down of Abd-B in LECs using Eip-71CD-Gal4 did not show any detectable phenotype (Figure 5B). Knock down of Abd-B in both HNCs and LECs driven by 71B-Gal4 and Pnr-Gal4, leads to anteriorization phenotypes in A5, A6 and A7 segments (Figure S6B and C). The Abd-B knockdown using 71B-Gal4 shows anteriorization phenotypes similar to esg-Gal4, however, it is milder than the esg-Gal4 (Figure S6B). This can be attributed to the lower, stochastic and late expression of 71B-Gal4 as compared to esg-Gal4 (Figure 2A1 and 3 and Figure S2). The Abd-B RNAi using Pnr-Gal4 shows complete loss of pigmentation on both sides of the dorsal midline in A5 to A6 segments indicating transformation of these cells into A4 like identity (Figure S6C). This phenotype is seen only in cells close to dorsal mid line, which very well corresponds to expression pattern of Pnr-Gal4 in the HNCs (Figure S3). These homeotic phenotypes seen in the case of Abd-B knockdown by esg, 71B and Pnr Gal4 drivers establish that expression of Abd-B in HNCs determines the identity of abdominal epithelia. The knockdown of Abd-B in LECs by using Eip-71CD-Gal4 does not give any phenotype (Figure 5B) indicating that expression of Abd-B in these cells is dispensable. From the results of abd-A and Abd-B RNAi, we conclude that the expression of abd-A is critical in both HNCs and LECs for development of the complete epithelia while expression of Abd-B is required in HNCs for their identity but dispensable in LECs.
Fig. 5. Expression of Abd-B is required in HNCs for the identity of the adult abdominal epithelia. 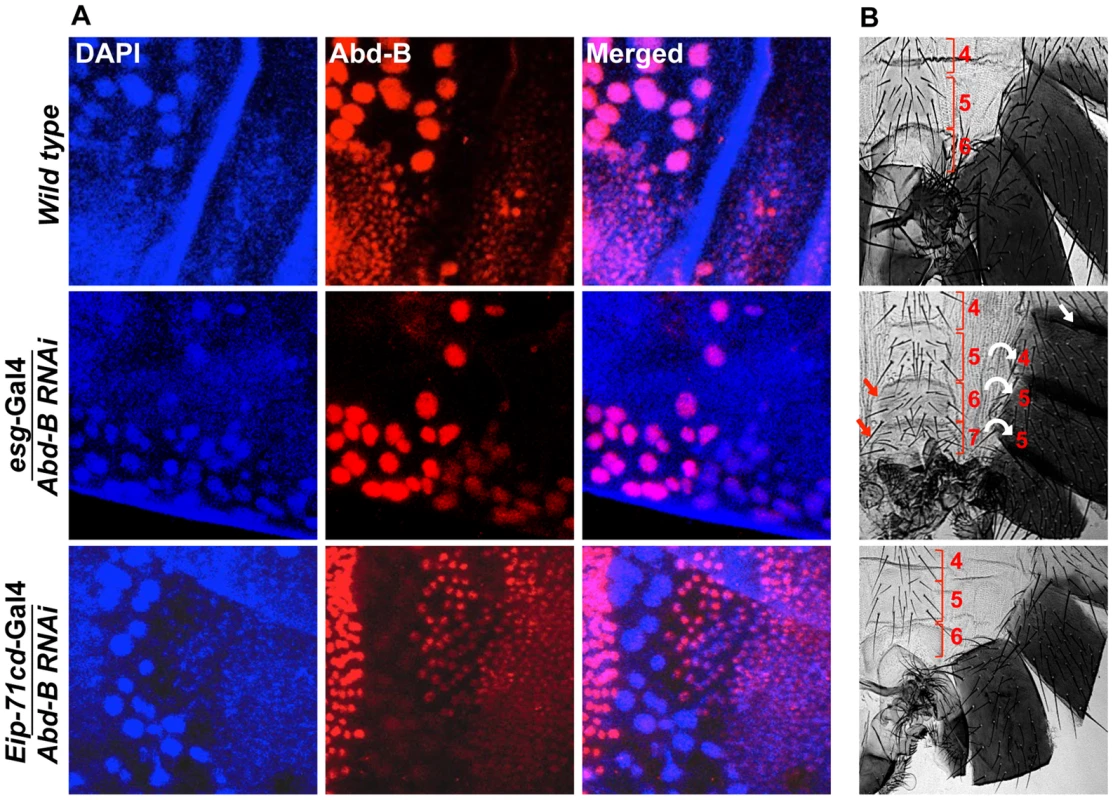
A) Abd-B expresses in both cell type HNCs and LECs in wild type (top panel). Knockdown of Abd-B using esg-Gal4 show HNCs specific loss of Abd-B protein (middle panel) while in the case of Eip-71CD-Gal4 we see LECs specific loss of Abd-B protein (lower panel). B) It shows cuticle preparations of the adult flies of the corresponding genotype in section A. HNC specific knockdown of Abd-B using esg-Gal4 shows homeotic transformation of A5, A6 and A7 into anterior segments. The A5 segment shows loss of pigmentation (white arrow), A6 segment shows sternite bristles and we see an extra A7 with sternite bristles (red arrow). Bracket sign marks the upper and lower limits of each segment. Knockdown of Abd-B in LECs using Eip-71CD-Gal4 does not show any phenotype (lower most panel). All the cuticles in this figure and subsequent figures are placed anterior at top and dorsal at right. abd-A activates wingless expression for development of abdominal epithelia
Recent studies show that higher level of Abd-B expression in A7 segment leads to loss of HNCs and LECs that results to elimination or smaller size of A7 segment in males and females, respectively [33], [34]. While analyzing expression of abd-A and Abd-B in abdominal epithelia, we observed that in the A7 segment expression of Abd-B is maximum and abd-A expression is very weak as compared to anterior segments. (Figure 1D). Thus we hypothesized that Abd-B being a posterior gene may be suppressing abd-A in A7 leading to the removal of A7 segment from the abdomen of males. To understand the Abd-B mediated suppression of abd-A, we analyzed the expression pattern of Abd-A in Fab-71 mutant and Abd-B RNAi driven by esg-Gal4. The Fab-71 mutant has a deletion of boundary element which leads to higher expression of Abd-B in A6 segment resulting in A6 to A7 transformation and thus loss of both the segments [36]. In wild type fly abd-A expresses weakly in A7 as compared to A5 and A6 (Figure 6A top panel), while in Fab-71 mutant we observed very weak expression of abd-A in both A6 and A7, suggesting that higher levels of Abd-B in A6 suppresses expression of abd-A in this segment (Figure 6A middle panel). To further confirm this observation, we knocked down Abd-B in HNCs of A7 using esg-Gal4 and observed increased expression of abd-A in this segment (Figure 6A lower most panel). The gain of abd-A expression in A7 is specific to HNCs and it was not seen in LECs, confirming Abd-B dependent suppression of abd-A. This establishes that expression of Abd-B similar to that in A7 segment, suppresses abd-A expression while lower expression in A5 and A6 does not effect abd-A expression. These experiments also clearly show that loss of abd-A expression correlates with the loss of abdominal segment and gain of abd-A correlates with the gain of abdominal segment in adults (Figure 6B). To test if abd-A is required and is enough for abdominal segment formation, we overexpressed abd-A in A7 segment using Abd-B-Gal4 and observed formation of an extra segment in males (Figure 7A lower panel) and a bigger A7 segment in females with 100% penetrance (Figure S7). This establishes that abd-A expression is required and is enough for adult abdominal epithelia formation during pupal development. And in A7, higher expression of Abd-B suppresses abd-A that leads to the loss of this segment in males and smaller segment in females.
Fig. 6. Higher expression of Abd-B suppresses abd-A expression in A7. 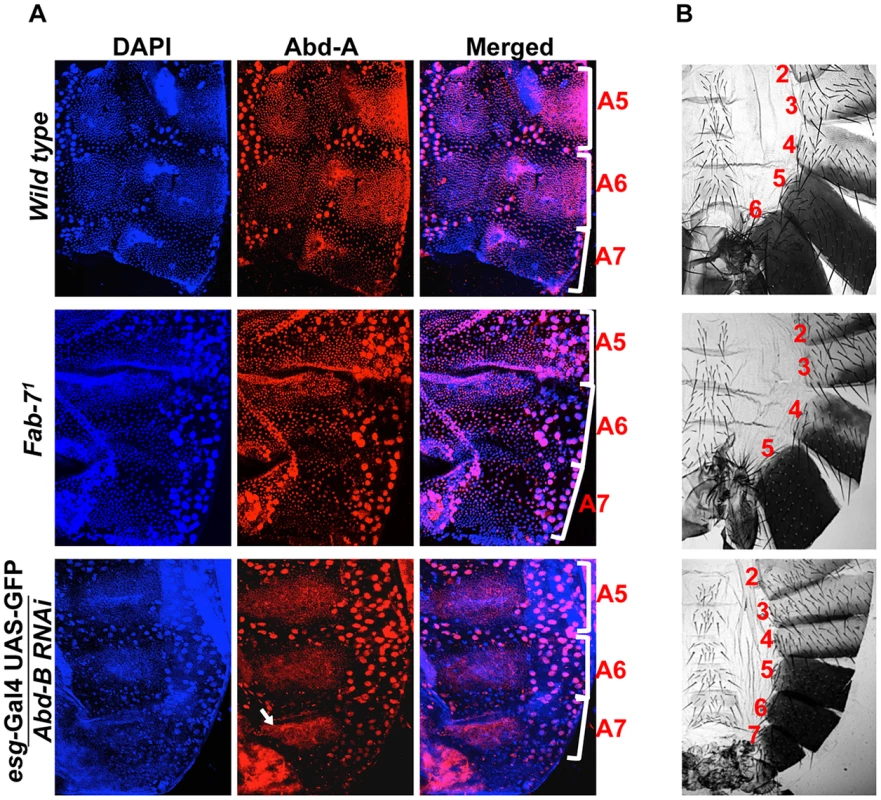
A) Wild type abdominal epithelia show very low expression of Abd-A in A7 in comparison to A5 and A6. The Fab-71 mutant show loss of abd-A in both A6 and A7 segment while in the case of Abd-B RNAi using esg-Gal4 we observed derepression of Abd-A expression only in HNCs of A7 segment (arrow). B) Cuticle preparations of adult flies show loss of A6 segment in Fab-71 mutant (middle panel) and homeotic transformation of A5, A6 and A7 into anterior segments on Abd-B RNAi with esg-Gal4. Fig. 7. Abd-A activates wingless for development of abdominal epithelia. 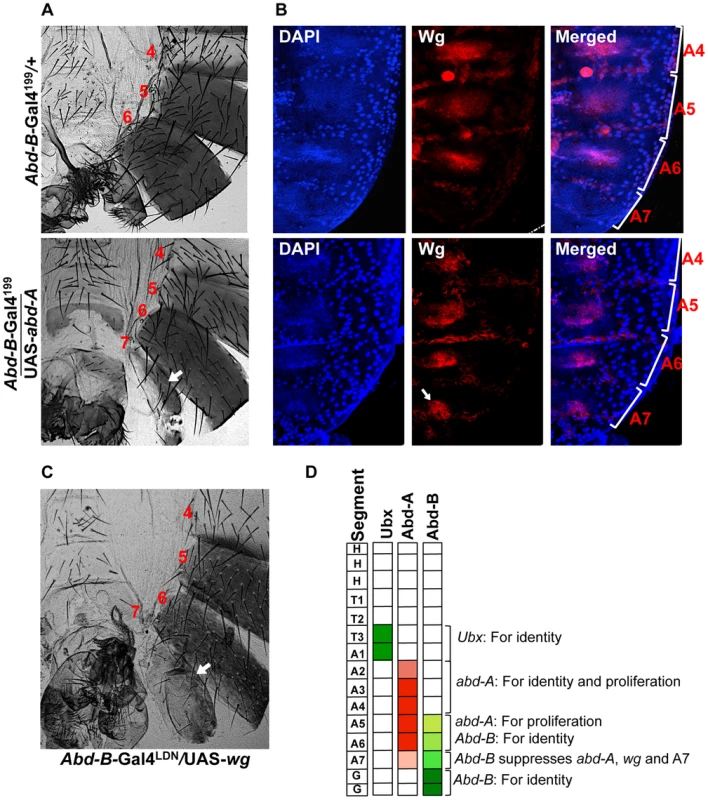
A) Over expression of abd-A using Abd-B-Gal4199 shows an extra segment in males (arrow in the lower panel) suggesting that abd-A expression is enough for abdominal epithelia formation. B) Over expression of abd-A in A7 using Abd-B-Gal4199 ectopically activates wg expression in A7 segment (arrow in lower panel). The genotypes are mentioned on left side of the A panel. C) Over expression of wg by Abd-B-Gal4LDN in A7 segment shows an extra segment. D) Summary of the hox gene expression and their role in abdominal epithelia development at pupal stage. Ubx expresses only in T3 and A1, the expression of abd-A in HNCs from A2 to A4 segment is required for both identity and proliferation while in A5 and A6 it is required for only proliferation. The expression of Abd-B in HNCs of A5 and A6 determines their identity while in A7 higher expression of Abd-B causes reduced size of this segment by suppressing the wingless pathway through suppression of abd-A. We further extended our study to understand how abd-A expression helps development of abdominal epithelia. Earlier studies have shown the role of Wingless (Wg) morphogen in regulating development of abdominal epithelia [37]–[39]. In A7 of male the expression of wg is known to be suppressed by higher Abd-B expression [34]. In this study we also observed that higher Abd-B levels suppress abd-A expression in HNCs of male A7 to promote elimination of this segment. This suggests that higher expression of Abd-B suppress both abd-A and wg expression in A7 for its elimination from male abdomen. We further wanted to understand if suppression of wg expression by Abd-B in A7 is through abd-A or independent of it. To study this, we analyzed the expression of wg in male A7 in the background of abd-A overexpression using Abd-B Gal4. Consistent with earlier expression we do not detect Wg morphogen in HNCs of male A7 (Figure 7B upper panel). The ectopic expression of abd-A leads to ectopic expression of wg in male A7 HNCs, clearly indicating that wg expression is positively regulated by abd-A (Figure 7B lower panel). This shows that wg expression in abdominal epithelial cells is activated by abd-A expression. To further prove that wg pathway is required for the formation of extra A7 segment we also did over expression of wg in A7 segment using Abd-B Gal4. We observed an extra A7 (Figure 7C) in all flies expressing wg under Abd-B-Gal4 similar to what we saw in over-expression of abd-A. This establishes that Abd-A protein activates wg expression for formation of abdominal epithelia. The higher expression of Abd-B in A7 of male induces elimination this segment by suppressing wg expression through suppression of abd-A expression (Figure 7D).
Discussion
The expression pattern of anterior Hox genes is often found to be overlapping with the posterior genes although the functional importance of such an expression pattern is unknown. In this study we show that the overlapping expression of Hox genes Ubx and abd-A during embryonic development of Drosophila becomes spatially non-overlapping in abdominal epithelia of pupa. The expression of Ubx is seen in T3 and A1 and not in posterior segments while the expression of abd-A is seen from A2 to A7, which overlaps with that of Abd-B from A5 to A7. Using UAS/GAL4 based RNAi and FRT/FLP based genetic mosaic techniques we show that abd-A is required in HNCs of A2 to A6 segment for their proliferation and suppression of Ubx expression to provide bigger size of the bristles. This suggests that abd-A is required in HNCs of A2 to A6 segments for development of adult epithelia with bigger bristle size. In contrast to this, in LECs abd-A expression is required for apoptosis, which allow proliferation of HNCs. Here, the interesting point is that these roles of abd-A in abdominal epithelia development are not limited only in the segments of exclusive expression (A2 to A4) but also in the segments where its expression overlaps with that of Abd-B (A5 to A6).
We further show that Abd-B expression is seen in both HNCs and LECs of A5 to A7 and functional studies suggested that it determines the identity of HNCs but it seems to be dispensable in the LECs. Knockdown of Abd-B in the HNCs of A5 to A7 segment of male leads to loss of pigmentation in A5 segment, bristles in sternite of A6 segment and formation of an extra A7 with bristles. This means that loss of Abd-B in HNCs of A5 leads to its transformation into A4 like features, transformation of A6 and A7 into A5. Loss of function results of abd-A and Abd-B RNAi bring out the function of both the genes in the HNCs of A5 and A6 during the development of adult abdominal epithelia. The two genes not only function together in same segment but also in same nuclei for their distinct roles. This is in contrast to the posterior dominance rule where an anterior gene does not function in the presence of a posterior Hox gene.
Interestingly, the situation in A7 is just the opposite, where a higher level of Abd-B suppresses abd-A expression. This suggests that abd-A can coexpress with Abd-B in the regions where Abd-B expression is less than that of A7. The over expression of abd-A in A7 shows an additional abdominal segment in males and bigger A7 segment in female, thus finally proving that Abd-A protein is required and sufficient for adult abdominal epithelia formation. This also implies that the down-regulation of abd-A in A7 by Abd-B protein is required only for the reduction in the size of abdominal segment. Earlier studies have shown additional male specific roles of Abd-B protein and sex-determination regulator Doublesex that regulate the elimination of A7 in males [33], [34]. The suppression of abd-A in A7 is seen as loss of Abd-A protein suggesting that posterior prevalence may be operating at transcriptional or post transcriptional level and not at post-translational level where concentration dependent interaction with common cofactor(s) was implicated in deciding the dominant function of posterior Hox gene [15]. Our study, with other published data, suggests that the expression of an anterior Hox gene in the domain of posterior Hox gene is not futile and that it has adopted new roles to play in such regions [16], [17].
This study and earlier work, taken together, explain how Hox genes of the bithorax complex control development of A1 to A7 segments of adult abdominal epithelia. The expression of Ubx, abd-A and Abd-B is required for the identity of A1, A2–A4 and A5–A7, respectively (Figure 7D). We show that the expression of abd-A is also required in A2 to A6 for the proliferation of HNCs and elimination of LECs. In A7, however, higher levels of Abd-B suppress epithelia formation by down regulating expression of abd-A. Interestingly, smaller size of A2 compared to A3 or A4 also correlates with the relatively lower level of expression of abd-A in A2 as compared to that in A3 or A4. This raises the possibility of the level of Abd-A determining the sizes of segments in adult fly. While the role of Hox genes in determining the identity of body segments is well established, our findings bring into light the collective role of Hox genes in determining the size, shape and identity of body segments. Our observations are in agreement with a recent study which shows that in embryonic CNS of Drosophila the expression of abd-A is not suppressed by Abd-B and that the two genes coexpress in same nuclei [40]. These observations suggest that posterior dominance rule operating between abd-A and Abd-B is tissues specific and not a universal phenomenon. Further studies will be required to understand the likely functional significance of such coexpression patterns of Hox genes in other tissues and animals as well. Much of what we understand about function of Hox genes is in the context of positional identity along the AP body axis. Our study suggests the need to explore the Hox code to understand development of organs where rules like posterior prevalence may not hold.
Materials and Methods
Fly stocks and culture
Flies were grown in standard cornmeal yeast extract medium at 25°C unless otherwise specified. We used following stocks during this study: CantonS (CS) as wild type strain, esg-Gal4 UAS-GFP (Nobert Perrimon), Abd-B-RNAi (Vienna Drosophila RNAi Centre), abd-A-RNAi, UAS-abd-A, UAS-Abd-B (Yacine Graba), McpH27 Fab-71, Fab-71, abd-AD24, Pnr-Gal4 (Francois Karch), UAS-miR-iab8-5p (Eric Lai) and Abd-B-Gal4LDN, Abd-B-Gal4199 (Ernesto Sánchez-Herrero), UAS-wg and UAS-Abd-B (LS Sashidhara) and w hsflp122;FRT82 abd-AD24/TM6 and w y hsflp122;FRT82 GFP/TM3, neoFRT82B Sb/TM6, 71B Gal4, Eip-71CD-Gal4 were procured from the Bloomington Drosophila Stock Centre.
Knockdown of Hox genes using UAS/Gal4 system
During this study we observed that abd-A RNAi line has multiple insertion of the P-element based RNAi vector in same chromosome. This gave us a clue that separating these P-elements would give us lines with less number of insertions and thus weaker abd-A RNAi effect. We recombined the abd-A RNAi carrying chromosome with the wild type chromosome and recovered lighter eye color line. In the case of Abd-B RNAi in McpH27 Fab-71 mutant background we recombined McpH27 Fab-71 mutant chromosome with third chromosome Abd-B RNAi to see the rescue in homozygous condition. In all RNAi and over expression experiments, we always used females from Gal4 lines while male from RNAi or over expression transgenic lines to employ maternal effect. All the phenotypic quantifications in the knockdown or over expression experiments is done using at least 40 larvae, pharates or adult flies of correct genotype. The pupae and larvae of correct genotypes were identified either by loss of Tubby marker present on balancer chromosome or Gal4 driven GFP expression.
Immunostaining of embryos and early pupal abdominal epithelia
10–14 hours old embryos of desired genotypes were used for immunostaining as per the published protocol [7]. Monoclonal antibodies; Anti Ubx (1∶50 dilution) and anti Abd-B (1∶10 dilution) were procured from Developmental Studies Hybridoma Bank, while polyclonal goat anti Abd-A (1∶200) antibody from Santa Cruz (27063). The secondary antibody tagged with HRP or fluorophore was used at 1∶200 dilutions. In the case of HRP chromogenic reaction was performed to stain the embryo and then CNS of the correct stage embryos were dissected out using fine needles and images were taken by using Zeiss AxioScop 2. For imaging of embryos stained with fluorophore conjugated secondary antibody we used confocal microscope.
For immunostaining of abdominal epithelial cells during early pupal development we followed the protocol described by Wang et al. [41]. Briefly, newly pupated pupae were collected on the basis of light color and staged as per the requirement. They were cut longitudinally into two halves from dorsal side with a sharp razor. Internal organs of cut pupae were removed by flushing 1XPBS using 20 µl pipette. Cleaned abdominal epithelia were fixed in 1XPBS +4% paraformaldehyde +0.2% dioxycholic acid for one hour. Tissue was further blocked in PBSB (1XPBS +1.0% BSA) for 3 hours and then incubated overnight with the antibody in same solution. The primary antibody anti-Ubx was used at 1∶20 dilution, anti-Abd-A at 1∶100 and anti-Abd-B at 1∶10 dilution. Further tissue was washed with PBSB and subjected to secondary antibody (1∶200) for 2 to 3 hours. After this the tissue was washed and mounted in anti-fade medium with DAPI (Vectashield). Images were taken by using confocal microscope.
Imaging of the pharates and adult abdomen
Pupae of correct stage and genotype were collected in microfuge tubes. They were washed with PBS to remove any adherent dirt and then fixed by boiling for 5 minutes in 1XPBS. The pupal case was removed gently with fine needles while in PBS to hatch the pharates [42]. The imaging of abdominal region of the fly was done by collecting the flies of interest and taking the pictures immediately or storing them at −30°C for later use. Wings and legs were cut if required for proper positioning and visualization of the abdomen. During acquisition of images light was given only from one side to avoid glittering of cuticle.
Live cell imaging
For live cell Imaging of HNCs we followed the protocol described by Nivon et al. [19]. Newly pupated milky white color pupae of correct genotype were collected and placed in moist chamber to avoid drying. Then pupae were aligned in correct orientation so that HNCs face towards the bottom of chamber with the help of halocarbon oil. Imaging of HNCs was done in gap of two hours using Zeiss multiphoton confocal. For calculation of the change in proliferation rate we did live cell imaging of 15 abd-A RNAi HNCs and 10 wild type HNCs. In this analysis we did not include HNCs that showed complete arrest in the proliferation.
Generation of mitotic clones
For making mitotic clones of abd-AD24 mutant, the mutant was recombined with FRT82 chromosome and recombinants were selected on G418 media. The hsflp; FRT82 abdAD24/TM6 females were crossed with FRT82/TM3 and FRT82 Sb/TM6 males separately. Progenies were given heat shock (37°C for 2 hours) at different stages of development for activation of the flipase. We at least screened 30 flies of correct genotype of each stage to score the percent of flies showing mitotic clones.
Preparation of fly cuticle
For making flat preparation of adult abdomen cuticle, flies of interest were collected and stored in 3∶1; ethanol:glycerol solution until use. Flies were heated at 90°C in 10% KOH for 10 minutes to dissolve all the tissue except cuticle. The cuticle was washed with PBS and stored in 50% glycerol. For mounting, abdomen of the fly was separated from the whole body by cutting between thorax and abdominal segment 1. Then the abdomen was cut from dorsal mid line to open the cuticle and remove all other tissues. Finally, cuticles were mounted with dorsal side facing up in 50% glycerol for imaging.
Supporting Information
Zdroje
1. PearsonJC, LemonsD, McGinnisW (2005) Modulating Hox gene functions during animal body patterning. Nat Rev Genet 6 : 893–904.
2. McGinnisW, GarberRL, WirzJ, KuroiwaA, GehringWJ (1984) A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell 37 : 403–408.
3. Sanchez-HerreroE, VernosI, MarcoR, MorataG (1985) Genetic organization of Drosophila bithorax complex. Nature 313 : 108–113.
4. ScottMP, WeinerAJ, HazelriggTI, PoliskyBA, PirrottaV, et al. (1983) The molecular organization of the Antennapedia locus of Drosophila. Cell 35 : 763–776.
5. DuncanI (1987) The bithorax complex. Annu Rev Genet 21 : 285–319.
6. WhiteRA, WilcoxM (1984) Protein products of the bithorax complex in Drosophila. Cell 39 : 163–171.
7. KarchF, BenderW, WeiffenbachB (1990) abdA expression in Drosophila embryos. Genes Dev 4 : 1573–1587.
8. CelnikerSE, KeelanDJ, LewisEB (1989) The molecular genetics of the bithorax complex of Drosophila: characterization of the products of the Abdominal-B domain. Genes & development 3 : 1424–1436.
9. MaedaRK, KarchF (2006) The ABC of the BX-C: the bithorax complex explained. Development 133 : 1413–1422.
10. DubouleD, MorataG (1994) Colinearity and functional hierarchy among genes of the homeotic complexes. Trends Genet 10 : 358–364.
11. KmitaM, van Der HoevenF, ZakanyJ, KrumlaufR, DubouleD (2000) Mechanisms of Hox gene colinearity: transposition of the anterior Hoxb1 gene into the posterior HoxD complex. Genes & development 14 : 198–211.
12. Gonzalez-ReyesA, UrquiaN, GehringWJ, StruhlG, MorataG (1990) Are cross-regulatory interactions between homoeotic genes functionally significant? Nature 344 : 78–80.
13. LamkaML, BouletAM, SakonjuS (1992) Ectopic expression of UBX and ABD-B proteins during Drosophila embryogenesis: competition, not a functional hierarchy, explains phenotypic suppression. Development 116 : 841–854.
14. MaciasA, MorataG (1996) Functional hierarchy and phenotypic suppression among Drosophila homeotic genes: the labial and empty spiracles genes. Embo J 15 : 334–343.
15. NoroB, LelliK, SunL, MannRS (2011) Competition for cofactor-dependent DNA binding underlies Hox phenotypic suppression. Genes Dev 25 : 2327–2332.
16. ForondaD, EstradaB, de NavasL, Sanchez-HerreroE (2006) Requirement of Abdominal-A and Abdominal-B in the developing genitalia of Drosophila breaks the posterior downregulation rule. Development 133 : 117–127.
17. MillerDF, RogersBT, KalkbrennerA, HamiltonB, HoltzmanSL, et al. (2001) Cross-regulation of Hox genes in the Drosophila melanogaster embryo. Mech Dev 102 : 3–16.
18. MadhavanMM, MadhavanK (1980) Morphogenesis of the epidermis of adult abdomen of Drosophila. Journal of embryology and experimental morphology 60 : 1–31.
19. NinovN, ChiarelliDA, Martin-BlancoE (2007) Extrinsic and intrinsic mechanisms directing epithelial cell sheet replacement during Drosophila metamorphosis. Development 134 : 367–379.
20. McGinnisW, KrumlaufR (1992) Homeobox genes and axial patterning. Cell 68 : 283–302.
21. CelnikerSE, SharmaS, KeelanDJ, LewisEB (1990) The molecular genetics of the bithorax complex of Drosophila: cis-regulation in the Abdominal-B domain. EMBO J 9 : 4277–4286.
22. IrishVF, Martinez-AriasA, AkamM (1989) Spatial regulation of the Antennapedia and Ultrabithorax homeotic genes during Drosophila early development. EMBO J 8 : 1527–1537.
23. BeachyPA, HelfandSL, HognessDS (1985) Segmental distribution of bithorax complex proteins during Drosophila development. Nature 313 : 545–551.
24. WangW, YoderJH (2012) Hox-mediated regulation of doublesex sculpts sex-specific abdomen morphology in Drosophila. Developmental dynamics: an official publication of the American Association of Anatomists 241 : 1076–1090.
25. WangW, TindellN, YanS, YoderJH (2013) Homeotic functions of the Teashirt transcription factor during adult Drosophila development. Biology open 2 : 18–29.
26. HayashiS, HiroseS, MetcalfeT, ShirrasAD (1993) Control of imaginal cell development by the escargot gene of Drosophila. Development 118 : 105–115.
27. BrandAH, PerrimonN (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 : 401–415.
28. KarchF, WeiffenbachB, PeiferM, BenderW, DuncanI, et al. (1985) The abdominal region of the bithorax complex. Cell 43 : 81–96.
29. SinghNP, MishraRK (2008) A double-edged sword to force posterior dominance of Hox genes. Bioessays 30 : 1058–1061.
30. TylerDM, OkamuraK, ChungWJ, HagenJW, BerezikovE, et al. (2008) Functionally distinct regulatory RNAs generated by bidirectional transcription and processing of microRNA loci. Genes Dev 22 : 26–36.
31. KarchF, GalloniM, SiposL, GauszJ, GyurkovicsH, et al. (1994) Mcp and Fab-7: molecular analysis of putative boundaries of cis-regulatory domains in the bithorax complex of Drosophila melanogaster. Nucleic Acids Res 22 : 3138–3146.
32. BantigniesF, RoureV, CometI, LeblancB, SchuettengruberB, et al. (2011) Polycomb-dependent regulatory contacts between distant Hox loci in Drosophila. Cell 144 : 214–226.
33. ForondaD, MartinP, Sanchez-HerreroE (2012) Drosophila Hox and sex-determination genes control segment elimination through EGFR and extramacrochetae activity. PLoS Genet 8: e1002874.
34. WangW, KiddBJ, CarrollSB, YoderJH (2011) Sexually dimorphic regulation of the Wingless morphogen controls sex-specific segment number in Drosophila. Proc Natl Acad Sci U S A 108 : 11139–11144.
35. ChenEH, ChristiansenAE, BakerBS (2005) Allocation and specification of the genital disc precursor cells in Drosophila. Developmental biology 281 : 270–285.
36. GyurkovicsH, GauszJ, KummerJ, KarchF (1990) A new homeotic mutation in the Drosophila bithorax complex removes a boundary separating two domains of regulation. EMBO J 9 : 2579–2585.
37. ShirrasAD, CousoJP (1996) Cell fates in the adult abdomen of Drosophila are determined by wingless during pupal development. Dev Biol 175 : 24–36.
38. KoppA, DuncanI, GodtD, CarrollSB (2000) Genetic control and evolution of sexually dimorphic characters in Drosophila. Nature 408 : 553–559.
39. KoppA, DuncanI (2002) Anteroposterior patterning in adult abdominal segments of Drosophila. Developmental biology 242 : 15–30.
40. GummallaM, GalettiS, MaedaRK, KarchF (2014) Hox gene regulation in the central nervous system of Drosophila. Front Cell Neurosci 8 : 96.
41. WangW, YoderJH (2011) Drosophila pupal abdomen immunohistochemistry. J Vis Exp 2 : 3139.
42. CurtissJ, HeiligJS (1995) Establishment of Drosophila imaginal precursor cells is controlled by the Arrowhead gene. Development 121 : 3819–3828.
Štítky
Genetika Reprodukčná medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 10- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



