-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
A Novel CCR5 Mutation Common in Sooty Mangabeys Reveals SIVsmm Infection of CCR5-Null Natural Hosts and Efficient Alternative Coreceptor Use
In contrast to HIV infection in humans and SIV in macaques, SIV infection of natural hosts including sooty mangabeys (SM) is non-pathogenic despite robust virus replication. We identified a novel SM CCR5 allele containing a two base pair deletion (Δ2) encoding a truncated molecule that is not expressed on the cell surface and does not support SIV entry in vitro. The allele was present at a 26% frequency in a large SM colony, along with 3% for a CCR5Δ24 deletion allele that also abrogates surface expression. Overall, 8% of animals were homozygous for defective CCR5 alleles and 41% were heterozygous. The mutant allele was also present in wild SM in West Africa. CD8+ and CD4+ T cells displayed a gradient of CCR5 expression across genotype groups, which was highly significant for CD8+ cells. Remarkably, the prevalence of natural SIVsmm infection was not significantly different in animals lacking functional CCR5 compared to heterozygous and homozygous wild-type animals. Furthermore, animals lacking functional CCR5 had robust plasma viral loads, which were only modestly lower than wild-type animals. SIVsmm primary isolates infected both homozygous mutant and wild-type PBMC in a CCR5-independent manner in vitro, and Envs from both CCR5-null and wild-type infected animals used CXCR6, GPR15 and GPR1 in addition to CCR5 in transfected cells. These data clearly indicate that SIVsmm relies on CCR5-independent entry pathways in SM that are homozygous for defective CCR5 alleles and, while the extent of alternative coreceptor use in SM with CCR5 wild type alleles is uncertain, strongly suggest that SIVsmm tropism and host cell targeting in vivo is defined by the distribution and use of alternative entry pathways in addition to CCR5. SIVsmm entry through alternative pathways in vivo raises the possibility of novel CCR5-negative target cells that may be more expendable than CCR5+ cells and enable the virus to replicate efficiently without causing disease in the face of extremely restricted CCR5 expression seen in SM and several other natural host species.
Published in the journal: A Novel CCR5 Mutation Common in Sooty Mangabeys Reveals SIVsmm Infection of CCR5-Null Natural Hosts and Efficient Alternative Coreceptor Use. PLoS Pathog 6(8): e32767. doi:10.1371/journal.ppat.1001064
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001064Summary
In contrast to HIV infection in humans and SIV in macaques, SIV infection of natural hosts including sooty mangabeys (SM) is non-pathogenic despite robust virus replication. We identified a novel SM CCR5 allele containing a two base pair deletion (Δ2) encoding a truncated molecule that is not expressed on the cell surface and does not support SIV entry in vitro. The allele was present at a 26% frequency in a large SM colony, along with 3% for a CCR5Δ24 deletion allele that also abrogates surface expression. Overall, 8% of animals were homozygous for defective CCR5 alleles and 41% were heterozygous. The mutant allele was also present in wild SM in West Africa. CD8+ and CD4+ T cells displayed a gradient of CCR5 expression across genotype groups, which was highly significant for CD8+ cells. Remarkably, the prevalence of natural SIVsmm infection was not significantly different in animals lacking functional CCR5 compared to heterozygous and homozygous wild-type animals. Furthermore, animals lacking functional CCR5 had robust plasma viral loads, which were only modestly lower than wild-type animals. SIVsmm primary isolates infected both homozygous mutant and wild-type PBMC in a CCR5-independent manner in vitro, and Envs from both CCR5-null and wild-type infected animals used CXCR6, GPR15 and GPR1 in addition to CCR5 in transfected cells. These data clearly indicate that SIVsmm relies on CCR5-independent entry pathways in SM that are homozygous for defective CCR5 alleles and, while the extent of alternative coreceptor use in SM with CCR5 wild type alleles is uncertain, strongly suggest that SIVsmm tropism and host cell targeting in vivo is defined by the distribution and use of alternative entry pathways in addition to CCR5. SIVsmm entry through alternative pathways in vivo raises the possibility of novel CCR5-negative target cells that may be more expendable than CCR5+ cells and enable the virus to replicate efficiently without causing disease in the face of extremely restricted CCR5 expression seen in SM and several other natural host species.
Introduction
HIV-1 emergence into the human population resulted from cross-species transmission of SIVcpz from chimpanzees (Pan troglodytes), which itself resulted from transmission and subsequent recombination of SIVs infecting primates on which chimpanzees prey [1], [2]. Similarly, both simian AIDS caused by SIVmac/smm in rhesus macaques (RM; Macaca mulatta) and HIV-2 infection of humans originated from cross-species transmission of SIVsmm from naturally infected sooty mangabeys (SM; Cercocebus atys) [3], [4], [5]. In marked contrast to pathogenic infections leading to AIDS in non-natural hosts, infection in natural host species including SM is typically non-progressive [6], [7], [8]. Importantly, the benign nature of SM infection in vivo is not due to overall restricted viral replication, as both nonpathogenic natural host and pathogenic nonnatural host infections are characterized by robust viremia [9], [10], [11], [12]. This observation indicates that immunodeficiency virus replication and pathogenesis are not inextricably linked. Thus, understanding natural host infection has become a high priority for identifying key features of infection in vivo that regulate pathogenesis and, potentially, identify opportunities to modulate disease apart from or in addition to suppressing overall virus replication through pharmacologic or immune mechanisms.
HIV and SIV entry into target cells is initiated by binding of the viral envelope glycoprotein (Env) to cell surface CD4, followed by structural changes that enable interactions with a seven transmembrane G protein coupled cell surface receptor that then triggers fusion. HIV-1 isolates use CCR5 or CXCR4 or both, and in vitro use other molecules infrequently. The restricted expression of CCR5 mainly on memory CD4+ T cells, but broader expression of CXCR4 on both memory and naïve subsets, is thought in part to underlie the accelerated disease progression seen in individuals in whom CXCR4-using HIV-1 variants emerge late in the course of infection [13], [14], [15], [16]. In contrast, SIV strains use CCR5 almost universally and very rarely use CXCR4. Sooty mangabeys express very low levels of CCR5 on their CD4+ T cells, a mechanism by which replication might be regulated in vivo and restrict transmission and pathogenesis [17], [18]. However, most strains of SIV use a number of other alternative coreceptors in in vitro assays, such as CXCR6 (STRL33), the orphan receptors GPR1 and GPR15, and several others [19], [20], [21]. Despite the efficient use of such alternative entry pathways by SIVmac and SIVsmm isolates in transfected cells, infection and cell targeting in vivo is generally thought to be dependent on CCR5 [22]. Notably, however, the proportion of CD4+ T cells depleted and/or infected at a given time in macaques and mangabeys may exceed the proportion of cells with detectable CCR5 expression, raising the possibility that other pathways in addition to CCR5 might be utilized [23], [24].
Although both natural and non-natural host infections result in high level virus replication, several distinguishing features provide clues as to possible causes for the distinct outcomes. It is long believed that in addition to CD4+ T cell destruction, pathogenesis involves an inability to effectively replenish these populations [25], [26]. In pathogenic rhesus macaque infection, damage to the CD4+ T central memory (Tcm) subpopulation appears to play a central role in the inability of infected animals to replenish CD4+ T effector and effector memory (Tem) cells depleted by infection [27]. It has been recently found that cell-associated viral loads in CD4+ Tcm are considerably lower in SM than RM, despite equivalent or higher Tem infection levels, which might enable better immune cell homeostasis in infected SM (G.S. and M.P; unpublished observations). Another difference is the presence of chronic generalized immune activation in infected humans and RM, whereas natural hosts display generalized immune activation during acute infection that then rapidly resolves [11], [28], [29], [30]. Chronic generalized immune activation may contribute to accelerated T cell turnover and ultimate depletion, and is believed to result in large part from translocation of gut microbial products due to gastrointestinal barrier damage during acute infection [31]. However, vigorous virus replication and extensive CD4+ T cell depletion in gut mucosal tissue occur in both natural and non-natural hosts [24], [32], [33], [34]. A potentially important difference is Th17 CD4+ T cells, which play a critical role in defense against bacteria at mucosal sites, and are lost in HIV-1 and SIVmac rhesus macaque infection but spared in infected natural hosts [35], [36], [37], [38]. Thus, the factors regulating CD4+ T cell subset targeting in vivo may be central to defining the outcome of infection in natural or non-natural hosts.
Interestingly, evolution appears to have favored mutations in the CCR5 gene that abrogate surface expression of this molecule. An allele containing a 32 base pair frameshift deletion in human CCR5 that abrogates cell surface expression (CCR5Δ32) is present at a frequency of 10% in the Caucasian population, resulting in about 18% heterozygous and 1% homozygous individuals. CD4+ cells from individuals homozygous for CCR5Δ32 are resistant to infection by CCR5-using HIV-1 isolates in vitro but permissive for strains that can use CXCR4, and the essential role for CCR5 in HIV-1 transmission and infection is demonstrated by the finding that individuals homozygous for CCR5Δ32 are highly resistant to infection [39], [40]. Furthermore, heterozygous individuals can be infected but show lower viral loads and slower disease progression, in association with lower levels of CCR5 expression [41], [42]. Red-capped mangabeys (RCM; Cercocebus torquatus) are the natural host of SIVrcm, and a 24 base pair in-frame deletion (CCR5Δ24) that also abrogates surface expression is present at an allelic frequency of 87% [43]. As a result, ≥70% of RCM are homozygous for the mutation and do not express CCR5, and the one known exception to exclusive CCR5 dependence by SIV in vivo is SIVrcm, which uses CCR2b for entry and cannot use CCR5. The same CCR5Δ24 mutant allele was also reported in two different populations of SM, which are closely related to RCM, but with a low allelic frequency of 4% and no animals homozygous for the allele were found [43], [44].
In this study, we identified a novel 2 base pair deletion and frameshift mutation in SM CCR5 (CCR5Δ2) that results in a lack of surface expression and coreceptor function. The mutation is present at a 26% allele frequency in the large Yerkes National Primate Research Center (YNPRC) SM colony, and together with the previously-described Δ24 allele results in 8% of animals lacking functional CCR5. However CCR5-null SM are susceptible to natural and experimental SIVsmm infection and exhibit robust viral replication. This is the first clear evidence for in vivo alternative coreceptor use by SIVsmm in its natural hosts, which provides an explanation for the efficient use of alternative coreceptors by the SIVsmm/mac family of viruses. This data also suggests that cell targeting and tropism in sooty mangabeys is linked to expression and use of both CCR5 and additional alternative entry pathways, and identifies a third example of convergent evolution resulting in nonfunctional mutant CCR5 alleles among primate species.
Results
Identification of a novel mutation in the sooty mangabey CCR5 gene
We amplified the CCR5 coding sequence from SM genomic DNA, cloned it into an expression vector and analyzed several clones by sequence analysis. Cloning from four independent PCR reactions amplifying smCCR5 from one animal (FVq) resulted in the identification of two distinct alleles in each of the PCR amplifications (Figure 1). One was a wild-type allele, similar to published smCCR5 sequences (smCCR5 wt), and the other was a novel allele containing a two base pair deletion at nucleotides 466 and 467 of the coding sequence (smCCR5Δ2), which corresponds to the fourth transmembrane domain (TM4) of the smCCR5 protein (Figure 1A). This deletion causes a frameshift that results in a predicted protein with 110 missense amino acids prior to termination at residue 265 (Figure 1B). In addition to this deletion, the smCCR5Δ2 allele contains two nucleotide substitutions compared with the wild-type allele identified. The first is a 436T>G substitution resulting in a L146V amino acid change, which is also found in several published wild-type smCCR5 sequences. The second nucleotide substitution is 538C>T, but is masked in CCR5Δ2 due to the frameshift.
Fig. 1. Sequence alignment of wild-type and mutant CCR5 genes. 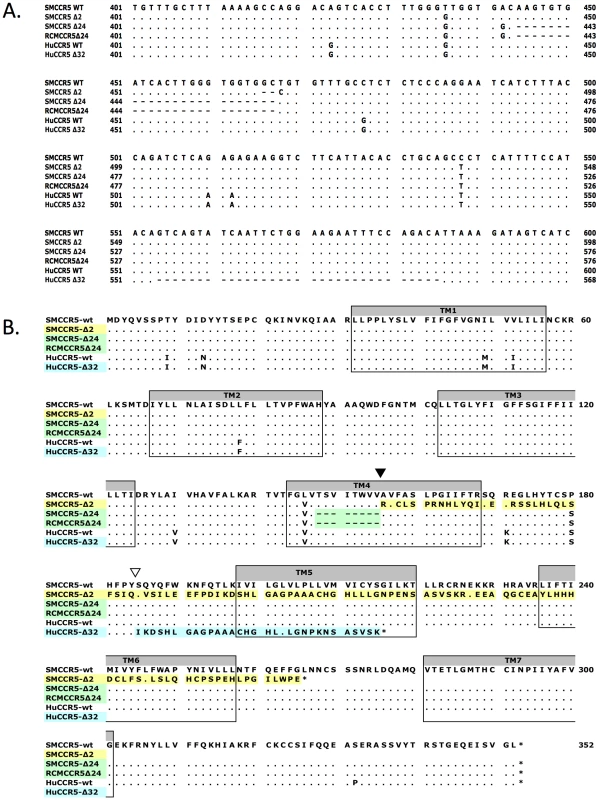
(A) Partial nucleotide sequence alignment and (B) predicted protein sequence alignment of the wild-type smCCR5 and smCCR5Δ2 alleles (GenBank HM246693 and HM246694), the CCR5Δ24 alleles previously described in sooty and red capped mangabeys (GenBank AF07473 and AAC62474), and human wild-type CCR5 and CCR5Δ32 alleles (GenBank DQ217934 and U66285). Residues identical to the wild-type smCCR5 molecule are indicated by ‘.’ and gaps are shown as dashes. Location of the frameshift resulting from the smCCR5Δ2 deletion in TM4 is indicated by an inverted black triangle while the huCCR5Δ32 frameshift is indicated by an inverted white triangle. Transmembrane domains are indicated by shaded boxes and mis-sense amino acid sequences resulting from mutations are highlighted. In addition to premature truncation of the protein, the mutation results in several charged amino acids predicted within TM4, as well as loss of both disulfide bonds (between the N-terminus and third extracellular loop (ECL), and between the first and second ECLs, respectively) that maintain secondary structure. These features suggest that the mutant protein is unlikely to be configured in a manner to allow proper membrane placement and either normal signaling or SIV/HIV entry coreceptor function.
There are several other mutations in primate CCR5 genes, including the well-studied 32 base pair frameshift mutation in human CCR5 (CCR5Δ32) [39], [40], [42] and a 24 base pair deletion (CCR5Δ24) that is common in RCM and also reported at low frequency in SM [43], [44]. Therefore, we examined the relationship between this SM CCR5Δ2 deletion and the Δ24 RCM/SM and human Δ32 deletions (Figure 1A & B). The smCCR5Δ2 deletion occurs in the same region of TM4, and overlaps the Δ24 deletion, which is characterized by multiple G-T-G repeats. In contrast, the human CCR5Δ32 deletion occurs approximately 90 bases downstream of smCCR5Δ2, within the second extracellular loop of the protein. Interestingly, the SM and RCM Δ24 alleles also contain the 436T>G and 538C>T substitutions seen smCCR5Δ2, which result in amino acids identical to those in human CCR5 at those respective sites (valine at position 146 and serine at position 180; Figure 1B).
Because the CCR5Δ24 mutation was previously reported to be present in animals at YNPRC [44], animals were screened for its presence by PCR and several Δ24 carriers were identified (Figure S1). We therefore generated a clone of the CCR5Δ24 coding region by PCR of genomic DNA from one heterozygous animal. Sequence analysis of this CCR5Δ24 clone was similar to the sequence previously described [43], [44], except for a non-coding 1026G>T substitution.
Cell surface expression of mutant smCCR5Δ2
Previous studies have shown that proteins encoded by the mutant human CCR5Δ32 and RCM/SM CCR5Δ24 are not expressed on the cell surface [40], [44]. To test whether the smCCR5Δ2 mutant gene gave rise to a protein expressed on the cell surface, we transfected 293T cells with wild-type and mutant SM CCR5 expression plasmids, along with human CCR5, and measured surface expression by flow cytometry. Staining utilized mAb 3A9, which cross-reacts with both human and SM CCR5 and, importantly, recognizes an epitope in the N-terminus that is upstream of the mutation and should be detected if the predicted protein were expressed.
As shown in Figure 2, surface expression of CCR5 was readily detected on cells transfected with SM or human wild-type CCR5. In contrast, neither the Δ2 nor Δ24 SM mutant CCR5 alleles gave rise to detectable surface expression. We have so far been unable to assess intracellular expression because of high nonspecific intracellular staining with 3A9 and other anti-N-terminal CCR5 antibodies in all cells tested so far (data not shown). From this data, we conclude that the frameshift mutation in smCCR5Δ2 results in a truncated protein that is not expressed on the cell surface. In addition, our observation that the CCR5Δ24 mutant allele is not expressed at the cell surface is consistent with previous reports that this deletion abrogates cell surface expression even though it is not a frameshift [43], [44]. We also asked if the mutant protein might have a dominant negative effect on wild-type CCR5 expression, but found no change in CCR5 staining if the wild-type CCR5 plasmid was co-transfected along with the Δ2 or Δ24 alleles (Figure S2).
Fig. 2. Surface expression of wild-type and mutant CCR5 variants in vitro. 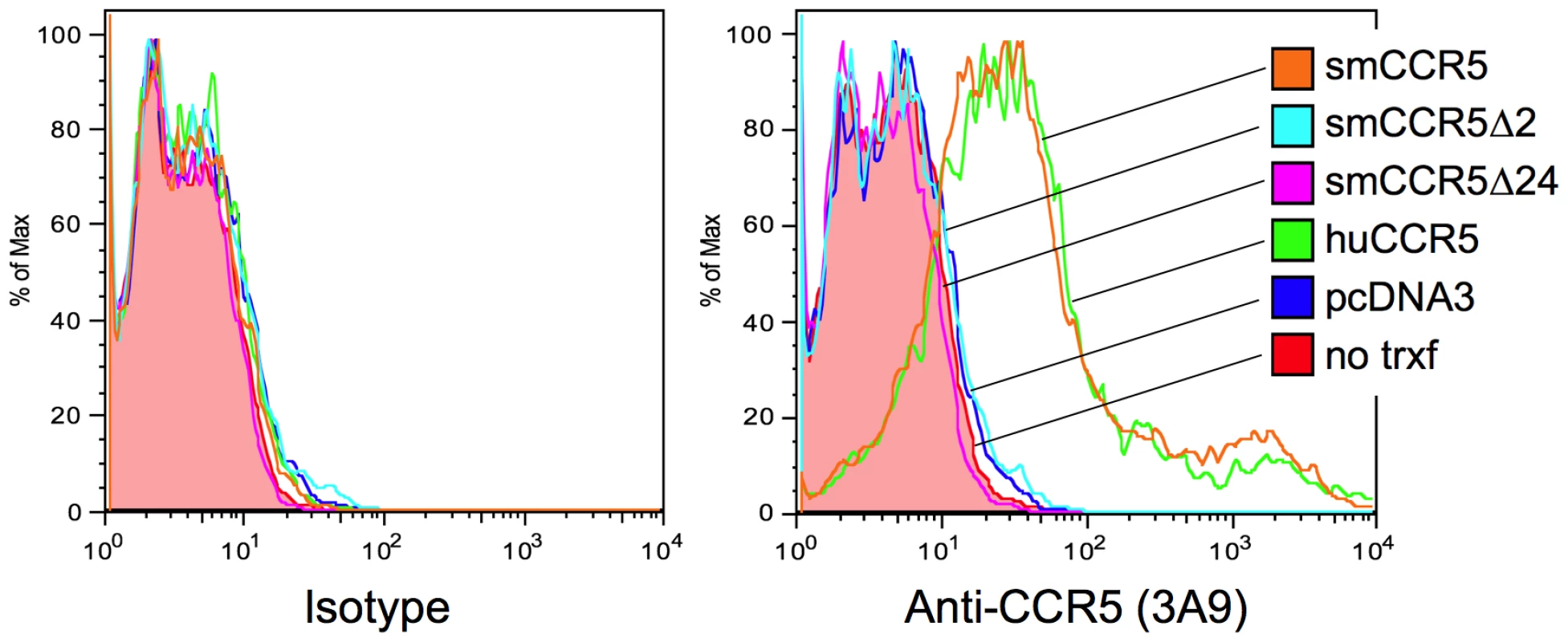
293T cells were transfected with expression plasmids encoding smCCR5 wild-type (orange), smCCR5Δ2 (light blue), smCCR5Δ24 (magenta) and huCCR5-wt (green), along with empty expression plasmid (dark blue). Untransfected 293T cells (shaded red) serve as controls. Cells were stained with an anti-CCR5 mAb (clone 3A9; APC-conjugated), which recognizes the N-terminal region of both human and sooty mangabey CCR5 (right panel), or an isotype-matched control antibody (left panel). Prevalence of the smCCR5Δ2 allele in the YNPRC sooty mangabey population
Next we determined the prevalence of the smCCR5Δ2 allele in SM housed at the Yerkes National Primate Research Center (YNPRC), which is the largest captive colony of SM in the world (n = 202). All animals were initially screened using a discriminatory PCR assay that specifically identifies the smCCR5 wild-type and Δ2 alleles (Figure S1A). Animals were also screened for the Δ24 allele using primers that discriminate Δ24 from Δ2 and wild-type alleles based on amplicon size (Figure S1B). Results were then verified by direct bulk sequencing of genomic DNA amplicons that confirmed the presence or absence of homozygous genotypes, or demonstrated frameshifting with sequence overlap in heterozygous animals (Figure S1C).
The result from this analysis is shown in Table 1. Five of six possible genotypes were identified: 50.5% of the SM carried two wild-type CCR5 alleles; 37.6% were heterozygous for wild-type and Δ2 alleles, and 4% of the SM were heterozygous for the wild-type and Δ24 alleles. Notably, 6.4% of the SM were homozygous for the smCCR5Δ2 allele, and 1.5% carried both Δ2 and Δ24 mutant alleles (Table 1). Thus, nearly 8% of animals carry two CCR5 mutant alleles encoding defective CCR5 proteins. We did not identify any SM that were homozygous for the Δ24 allele.
Tab. 1. Genotypic frequencies in Sooty Mangabeys at YNPRC. 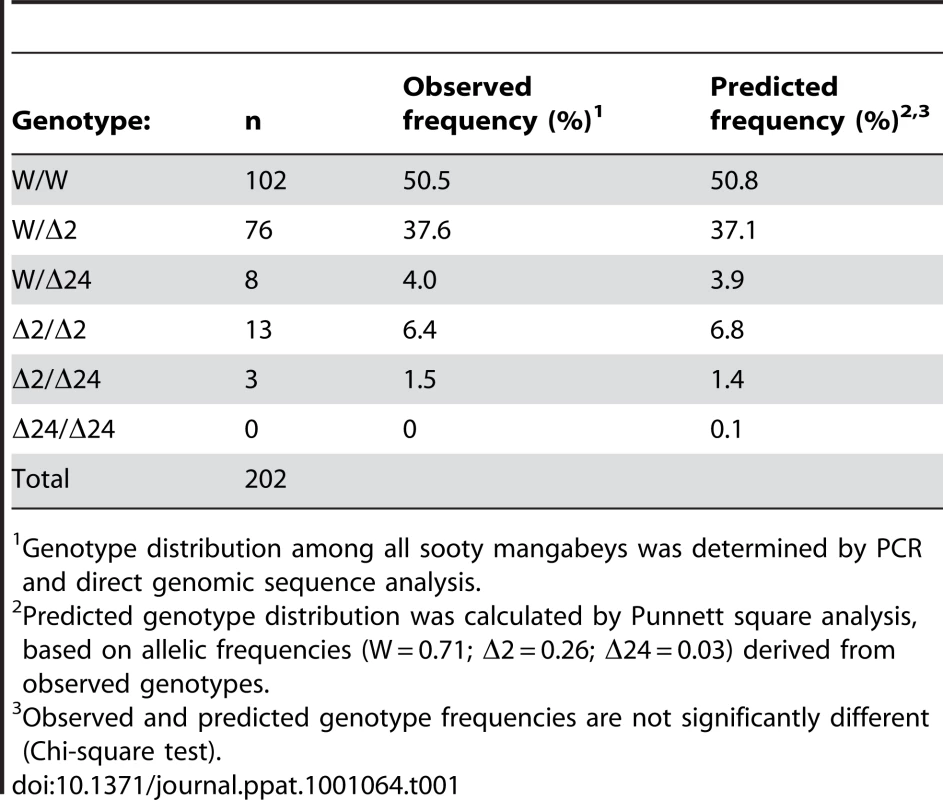
Genotype distribution among all sooty mangabeys was determined by PCR and direct genomic sequence analysis. In this SM population, analysis of allelic frequencies showed 26% of alleles carried the novel Δ2 deletion, 3% carried the Δ24 deletion, and 71% were wild-type. Of note, the 3% frequency we found for the Δ24 allele is similar to the 4% allelic frequency described 12 years ago among SM housed at YNPRC [44]. We then used the allelic frequencies to calculate a predicted genotype distribution (Table 1 and Table S1). There was close agreement between predicted and observed genotypes (p = ns; Chi-square test), suggesting that the CCR5 alleles are in equilibrium in this population (of which ∼60% are SIV-infected), without evidence of selective pressure favoring or disfavoring any of the genotypes.
Prevalence of mutant CCR5 alleles in other captive and wild African sooty mangabey populations
The YNPRC colony is the largest population of SM in the US and the close match between predicted and observed CCR5 genotype distributions suggested an absence of selective pressure for or against any specific genotype. However, we wished to determine the frequency with which these alleles and genotypes were present in other SM populations, so we analyzed genomic DNA obtained from 29 animals housed at the Tulane National Primate Research Center (TNPRC). Of note, many of the monkeys that founded the TNPRC colony originally came from YNPRC in the 1980s, but have been housed and bred separately since then. In this smaller population the Δ2 allele was present at a frequency of 19% and the Δ24 allele had a frequency of 5%. As shown in Table 2 and Table S2, the observed genotype frequencies in this population also do not differ from those predicted by allele frequencies.
Tab. 2. Observed and predicted genotype frequencies in Sooty Mangabeys housed at the TNPRC1. 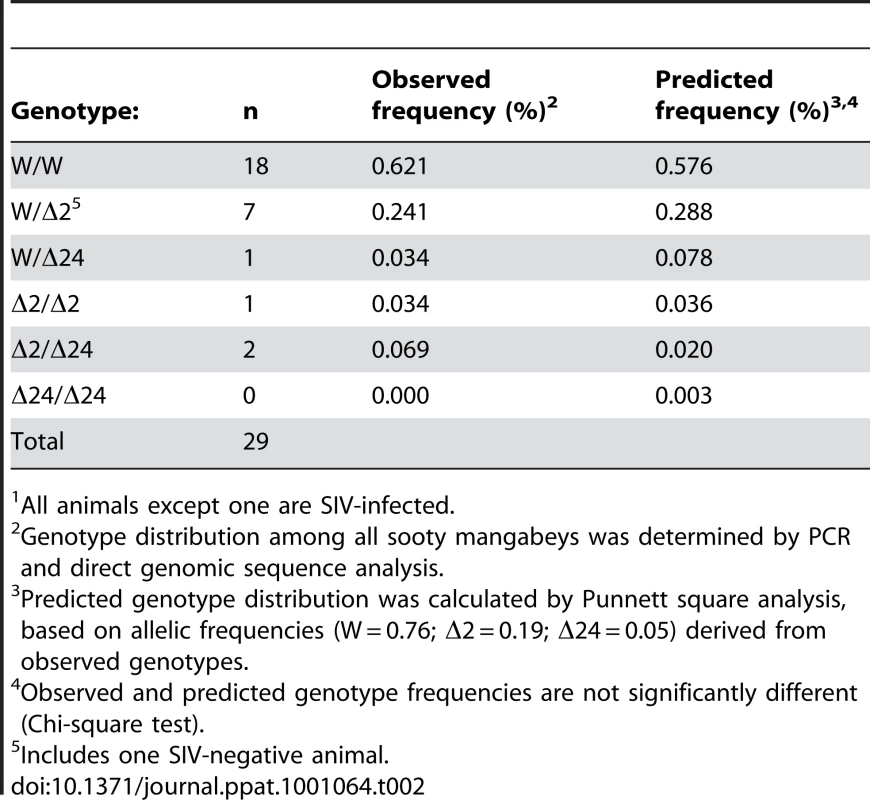
All animals except one are SIV-infected. We also asked whether the CCR5Δ2 allele was present in SM in Africa. For this analysis we amplified CCR5 genes from fecal-derived host DNA samples from 33 wild-living animals in the Tai forest of Cote d'Ivoire [45]. Five animals carried both the CCR5Δ2 and wild-type alleles, and in one animal only the Δ2 allele was detected. An additional 2 animals carried both Δ24 and wild-type alleles, while the remaining animals revealed only wild-type sequences. Because this analysis utilized fecal DNA containing limiting quantities of host DNA, we can only be certain that both alleles were captured if animals were found to be heterozygous. Thus, while it is not possible to determine a precise allele frequency, these genotypes suggest a minimum allele frequency of 9% for CCR5Δ2 and 3% for CCR5Δ24 in this population. This result indicates that the Δ2 allele is also present in wild-living SM in Cote d'Ivoire, although likely at a lower frequency than in captive animals at YNPRC.
Expression of CCR5 on SM CD4+ and CD8+ T cells ex vivo
Since overexpression studies in transfected cells suggested that neither this common smCCR5Δ2 nor the less common Δ24 proteins are expressed on the cell surface, we examined the relationship between genotypes and CCR5 expression on primary SM CD4+ and CD8+ T cells. To address this point, we analyzed CCR5 expression data that was available from animals housed at the YNPRC collected during periodic surveys between 2004 and 2009, focusing on uninfected animals, and grouped individuals according to their CCR5 genotypes. Since neither Δ2 nor Δ24 CCR5 alleles express following transfection, the two mutations were combined for the purposes of this analysis into a homozygous wild-type, heterozygous, and homozygous deletion allele groups (Figure 3).
Fig. 3. CCR5 surface expression on sooty mangabey CD4+ and CD8+ T cells ex vivo. 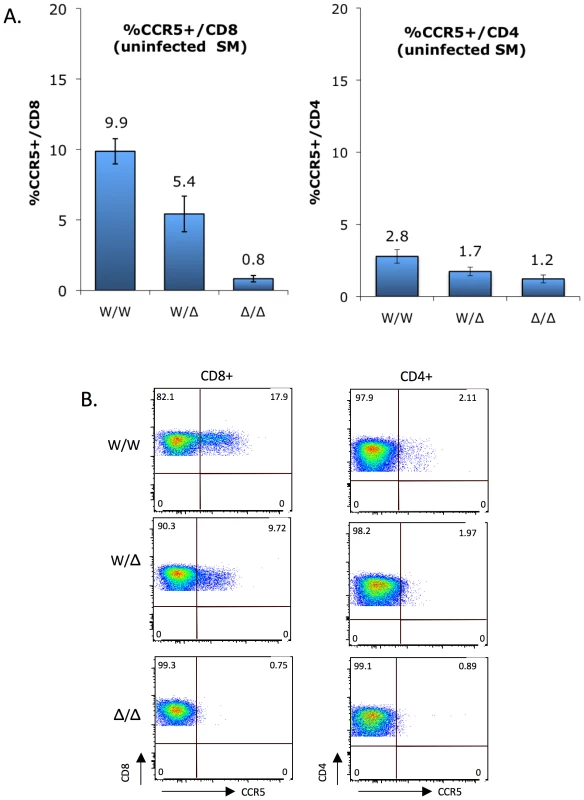
(A) CCR5 staining on CD8+ T cells (left) and CD4+ T cells (right) of uninfected sooty mangabeys was carried out between 2004 and 2009, and analyzed according to genotype groups (mean ± SEM): CCR5 wild-type (W/W; n = 38), heterozygous (W/Δ; n = 34) and homozygous mutant (Δ/Δ; n = 7). Because both Δ2 and Δ24 alleles are functionally null, they were combined for this analysis. CCR5 expression by CD8+ T cells differs significantly between genotype groups (p<0.0001 by Kruskal-Wallis test), while the trend for CD4+ T cells does not reach statistical significance (p = ns). (B) FACS plots showing CCR5 staining on CD4+ and CD8+ T cells from representative homozygous wild-type, heterozygous and homozygous mutant animals. As previously reported [18] and as shown in Figure 3, CCR5 expression is markedly greater on SM CD8+ T cells than CD4+ T cells. In both populations there was a gradation in the percentage of cells staining positive for CCR5 that correlated with CCR5 genotype status. The difference was particularly evident for CD8+ T cells, and the percentage of CCR5+/CD8+ T cells was 9.9%, 5.4% and 0.8% for wild-type, heterozygous and homozygous mutant groups, respectively (Figure 3A; p<0.0001 by Kruskal-Wallis test). This finding indicates that the CCR5Δ2 genotype acts as a determinant of CCR5 expression on CD8+ T cells. There was also a strong linear relationship between wild-type gene dosage and CCR5 expression for CD8 cells (R2 = 0.99996; p = 0.009 by Pearson's 2-tailed correlation coefficient), consistent with the absence of a dominant negative effect by the mutant alleles in transfected cells (Figure S2). A trend was also evident for CCR5 staining on CD4+ T cells among the three groups (2.8%, 1.7% and 1.2% in the wild-type, heterozygous and homozygous mutant groups, respectively), which did not reach statistical significance (p = ns by Kruskal-Wallis test) but is consistent with the notion that, although overall very low, CCR5 expression on CD4+ T cells is also regulated by CCR5 genotype.
Representative FACS plots showing CCR5 expression on CD4+ and CD8+ T cells from a homozygous wild-type, heterozygous and homozygous mutant animal are shown in Figure 3B. We think the low level (∼1%) of cells within the CCR5 gate for homozygous mutant animals likely represents background staining, rather than low levels of N-terminal expression given the result of transfection studies (Figure 2). Unfortunately, antibodies available that are directed at other epitopes in human CCR5 do not recognize SM CCR5.
CCR5-null genotype does not protect sooty mangabeys from SIVsmm infection in vivo
In humans, the CCR5Δ32 homozygous genotype provides powerful protection against HIV-1 infection [39], [40], [42]. Therefore, we asked if animals homozygous for CCR5-null alleles were infected by SIVsmm. In order to more properly assess natural susceptibility, we restricted this analysis to YNPRC animals that were naturally infected (n = 120) and those with documented SIV-negative status (n = 72), and excluded animals in the colony known to have been experimentally infected (n = 10).
As shown in Table 3, we found that among SM with the CCR5 homozygous wild-type genotype, 65% were naturally infected while 35% were uninfected, and 62% of CCR5 heterozygous animals were infected and 38% were uninfected. Unexpectedly, among animals homozygous for CCR5-null alleles (n = 14), 50% were naturally infected and 50% were seronegative. Thus, animals lacking functional CCR5 genes are susceptible to natural SIVsmm infection. The slightly lower prevalence of SIV infection among animals in the CCR5-null group was not statistically significant (p = ns; Chi-square test). We also compared the distribution of genotypes within the SIV-negative and naturally-infected SIV populations (Table 4). Similarly, there were no significant differences in genotype distribution between the SIV serostatus groups (p = ns for each genotype group; 2-sample proportions test), although a slightly lower proportion of homozygous CCR5 mutant animals was seen in the infected animals compared with SIV-negative animals (5.8% vs. 9.7%; p = ns). Therefore, the CCR5-null genotype does not prevent natural acquisition of SIVsmm infection. Furthermore, neither heterozygosity nor homozygous null genotype appears to significantly influence SM susceptibility to SIVsmm infection in vivo, and while we cannot absolutely rule out a small effect, any protection that might be afforded by CCR5-null status would be slight. This result stands in marked contrast to the profound protective effect of CCR5Δ32 homozygosity in humans.
Tab. 3. Prevalence of naturally-acquired SIV infection among YNPRC sooty mangabeys based on CCR5 genotype. 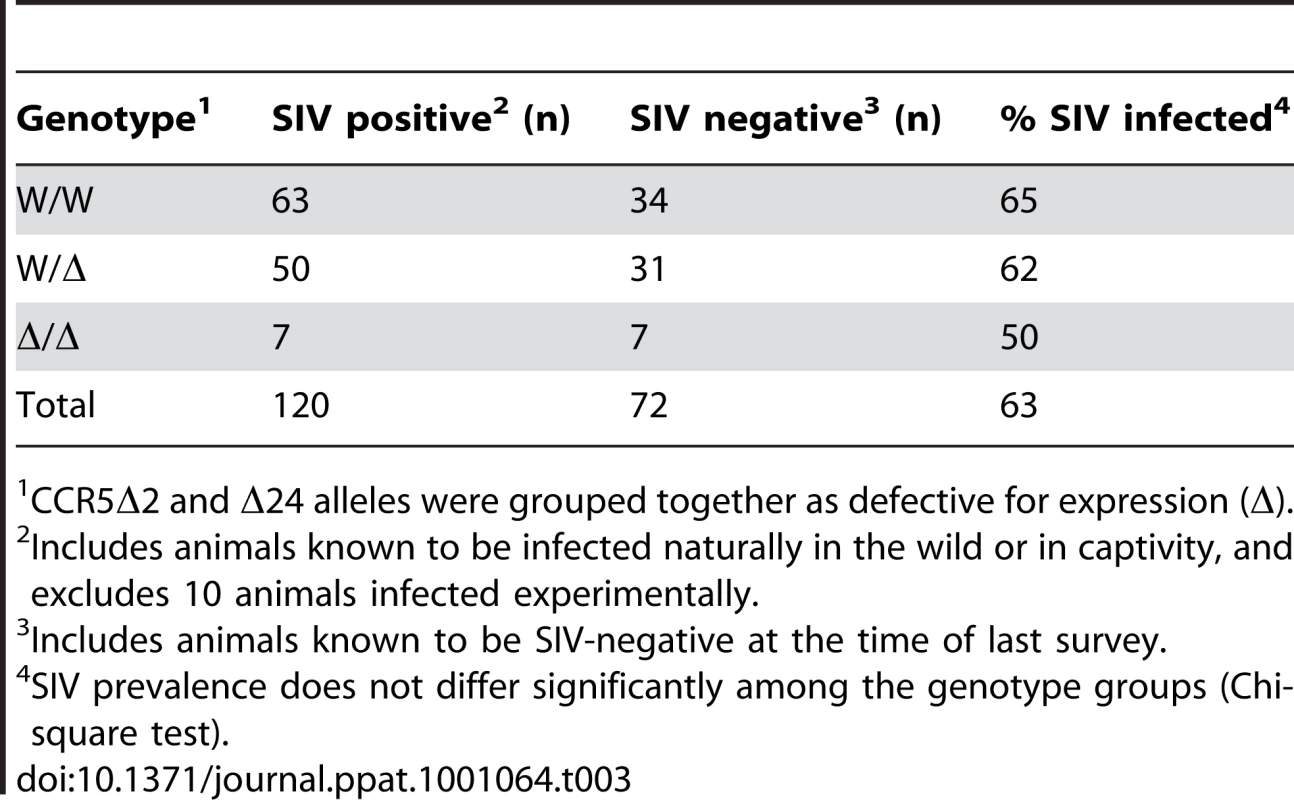
CCR5Δ2 and Δ24 alleles were grouped together as defective for expression (Δ). Tab. 4. Genotypic distribution among YNPRC sooty mangabeys based on SIV infection status. 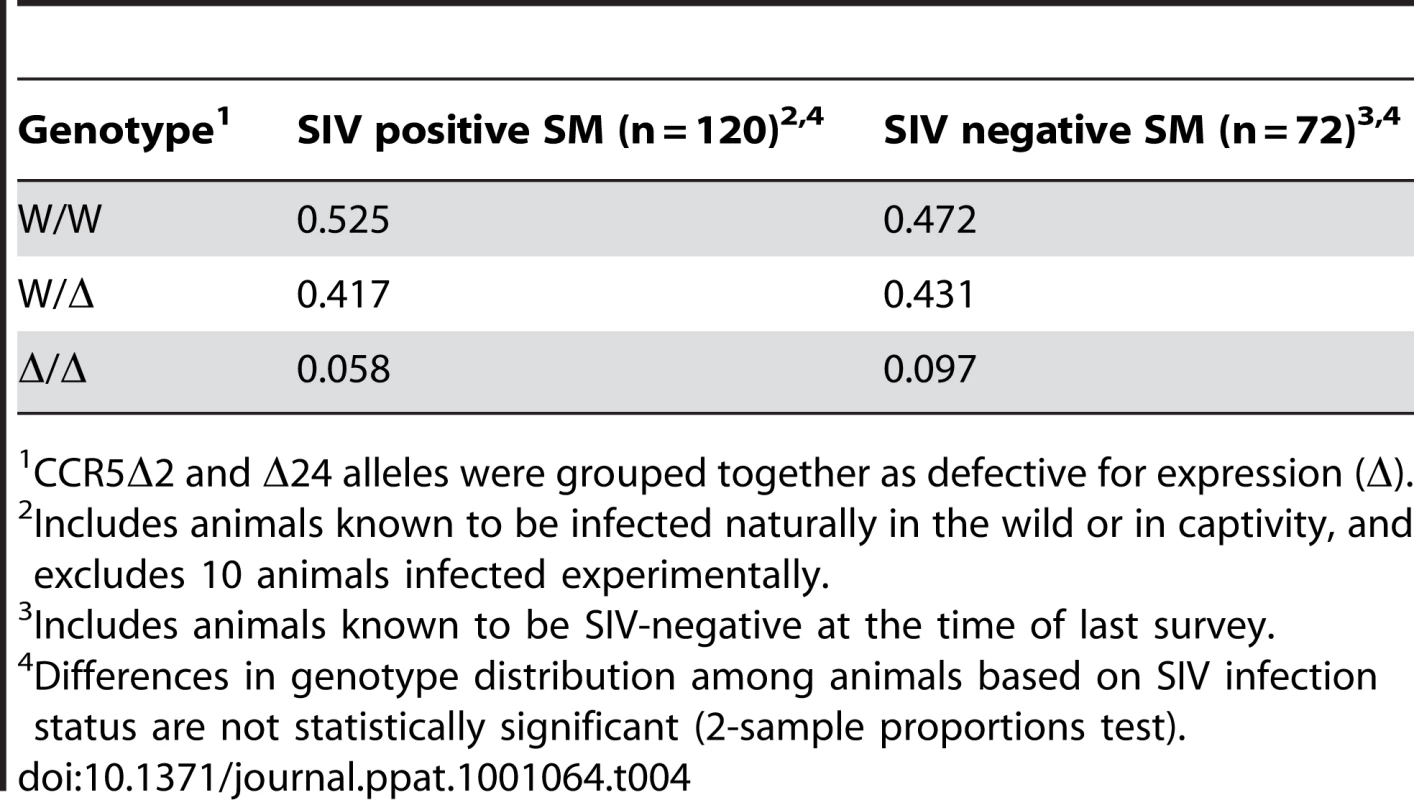
CCR5Δ2 and Δ24 alleles were grouped together as defective for expression (Δ). We also determined the genotypes of 10 animals at YNPRC that were infected experimentally. Five of these animals were homozygous for the CCR5 wild-type gene, three were heterozygotes (all W/Δ2) and two were homozygous for smCCR5Δ2. Furthermore, all but one of the TNPRC animals studied are SIVsmm-infected, including a mix of both natural infections and experimental inoculation done in earlier decades. Among the infected animals were three homozygous CCR5-null animals, while the one uninfected animal was heterozygous (W/Δ2). Therefore, SM naturally deficient in CCR5 expression are susceptible to experimental as well as natural SIVsmm infection, confirming that non-CCR5 entry pathways can mediate SM natural host infection.
Influence of smCCR5 genotype on plasma viral loads and CD4 counts in SIVsmm-infected SM
We next asked if CCR5 genotype affected plasma viral loads in infected animals. Based on previously collected data, the log10 viral load (mean ± SEM) was calculated for animals in each genotype group; for animals with multiple data points available, their mean viral load (log10) was used (Figure 4). This analysis showed robust viral loads in all genotype groups, with a modest but statistically significant gradient in VL dependent on the presence of a wild-type CCR5 allele (p = 0.005 by Kruskal-Wallis test). Animals possessing two wild-type alleles exhibited the highest viral load (4.83±0.10 log10), heterozygotes showed an intermediate level (4.65±0.10 log10), and infected animals with the CCR5-null genotype had the lowest VL (4.37±0.15 log10). Thus, there is approximately 0.5 log10 difference in viral load in animals with two wild-type compared with two CCR5-null alleles. The two important points here are that homozygous mutant animals have vigorous viral replication despite lacking functional CCR5, and simultaneously exhibit a small but significant difference in plasma viral load associated with increasing CCR5 gene dosage.
Fig. 4. SIV plasma viral load measurements in infected sooty mangabeys between genotype groups. 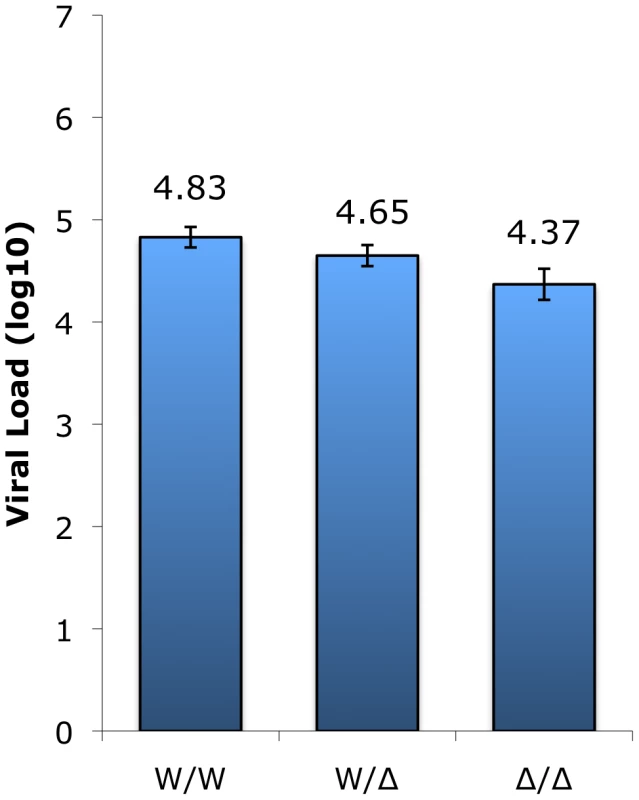
Plasma viral load measurements (VL log10; means ± SEM) collected in surveys carried out between 2004 and 2009 of infected animals in the wild-type (n = 60), heterozygous (n = 49), and homozygous mutant (n = 7) genotype groups. The Δ2 and Δ24 alleles were combined for this analysis. The difference in VL between homozygous wild-type and CCR5-null genotype groups (W/W vs. Δ/Δ) is statistically significant (p<0.05; Dunn's multiple comparison test) whereas the differences for the heterozygous group (W/Δ vs. W/W; W/Δ vs. Δ/Δ) does not reach statistical significance (p = ns; Dunn's multiple comparison test). CD4 counts remain stable during chronic SIVsmm infection in the vast majority of animals, but some exceptions have been noted [46], [47], so we next asked if SIV-infected animals exhibit differences in CD4 counts depending on their CCR5 genotype. However, there was no significant difference in CD4+ T cell levels among the genotype groups, whether assessed based on absolute counts (Figure S3A; p = ns, Kruskal-Wallis) or on the basis of CD4 percentage (Figure S3B; p = ns, ANOVA). These results suggest that CD4+ T cell levels are maintained similarly in SIV-infected SM regardless of CCR5 genotype.
smCCR5Δ2 does not support SIV entry in vitro
We then asked if there was any chance that the smCCR5Δ2 mutant allele, when expressed along with CD4, could support SIV infection in vitro. We considered it unlikely but thought it was necessary to test directly given the staining patterns by CCR5 mAb 3A9 in Δ2 homozygous primary mononuclear cells (Figure 3). We also considered it important to test using SIVsmm from a CCR5Δ2 homozygous infected animal, in case viral adaptation might have enabled use of an N-terminal region alone. Therefore, we generated pseudotype virions carrying Env glycoproteins cloned directly from plasma virus of an SIVsmm-infected CCR5Δ2 homozygous animal (FNp), along with pseudotypes carrying Envs cloned from plasma of an infected wild-type animal (FFv). Use of plasma virus ensured that these Envs were derived from actively replicating virus. Target 293T cells were co-transfected with CD4 plus plasmids encoding wild-type smCCR5, smCCR5Δ2, human CCR5 or an empty vector as a control, then infected with the primary SIVsmm pseudotypes. Of note, pseudotype virions carrying the FNp 5.1 Env were considerably less infectious than the other Envs and very large amounts of this virus were required to achieve equivalent infectious inocula, which generally resulted in high levels of background for this Env.
As shown in Figure 5, wild-type smCCR5 and human CCR5 support infection of all SIVsmm variants to similar levels. In contrast, the smCCR5Δ2 allele does not support infection by any of the viruses. Importantly, CCR5Δ2 does not function as a coreceptor for Env variants from CCR5-null animals. Thus, SIVsmm infections in CCR5-null animals are mediated through pathways independent of CCR5.
Fig. 5. Mutant smCCR5Δ2 does not support SIV infection in vitro. 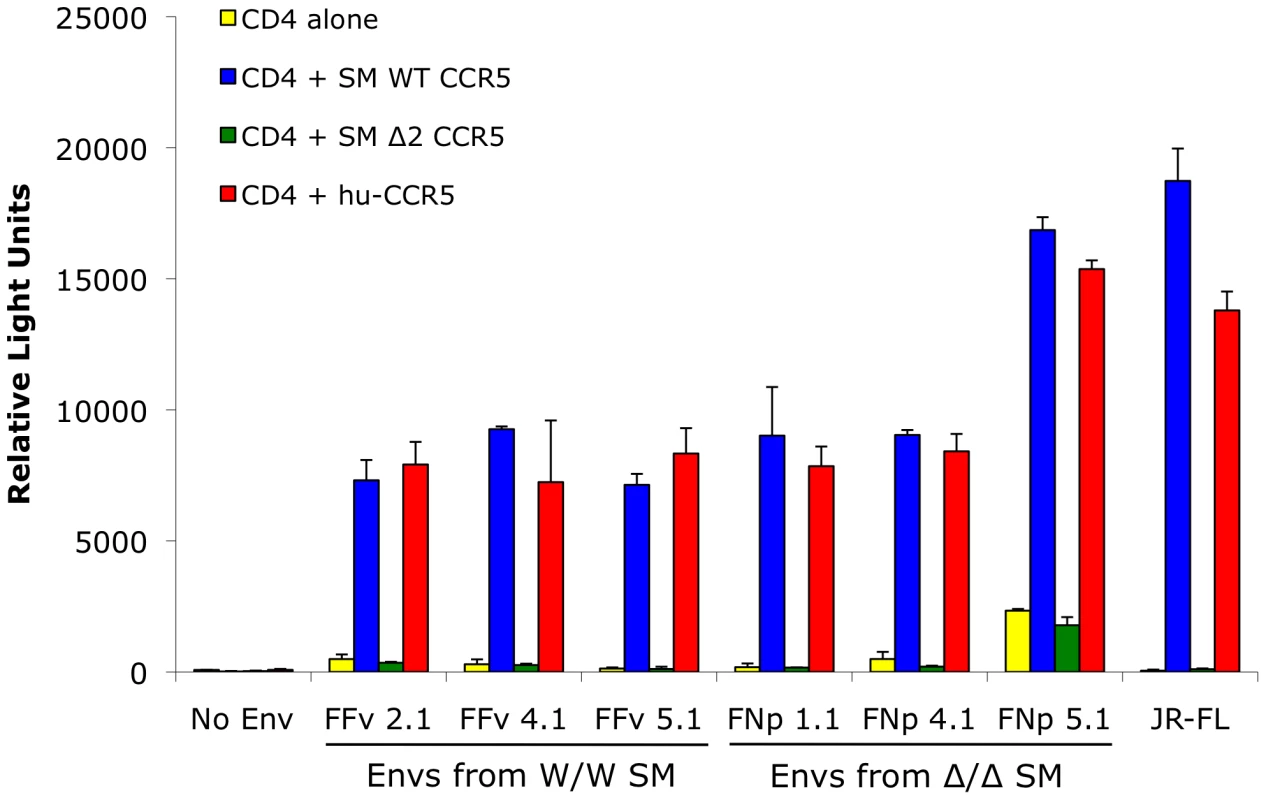
293T cells were transfected with CD4 alone (yellow bars) or in combination with wild-type smCCR5 (blue bars), smCCR5Δ2 (green bars) or wild-type human CCR5 (red bars). Target cells were infected with luciferase-expressing pseudotype virions carrying Env glycoproteins that were cloned from plasma of two SIVsmm-infected sooty mangabeys (FFv: W/W SM; FNp: Δ2/Δ2 SM). Pseudotypes carrying the R5-tropic HIV-1 Env JRFL and virions lacking envelope glycoproteins served as controls. Infection was measured by relative light units (RLU) in cell lysates 3 days after infection (mean ± SD). Use of alternative coreceptors by SIVsmm isolates
The data presented here indicates that SIVsmm must be able to use entry pathways other than CCR5 for replication in vivo. It is long known that many SIV strains use a number of alternative coreceptors in addition to CCR5 in vitro, although these viruses rarely use CXCR4. We therefore tested the ability of SIVsmm envelopes to mediate infection through human CCR2b, CCR3, CCR8, GPR1, GPR15 (BOB), CXCR6 and CXCR4. This analysis employed the uncultured SIVsmm envelope glycoproteins cloned from plasma of the CCR5-null (FNp) and homozygous wild-type (FFv) infected animals.
As shown in Figure 6, all SIVsmm envelope glycoproteins tested mediated entry into cells expressing CD4 in conjunction with GPR15 and CXCR6, while GPR1 was also used but less efficiently. In contrast, none of the SIVsmm envelope glycoproteins could use CXCR4 as a coreceptor, nor CCR3 or CCR8 (data not shown). Interestingly, SIVsmm envelope glycoproteins failed to use CCR2b, an entry pathway employed by SIVrcm in RCM that typically lack CCR5 due to a high prevalence of the Δ24 mutation [43]. Furthermore, the patterns of alternative coreceptor use were similar for envelope glycoproteins derived from CCR5-null and CCR5 wild-type animals, indicating that alternative coreceptor utilization is a feature shared by SIVsmm regardless of whether the host animal expresses CCR5. While absolute luciferase production varied among experiments, GPR15 and CXCR6 typically supported levels of infection similar to that mediated by CCR5 (Figure S4), although transfected targets likely represent maximum levels of potential utilization relative to primary cells that would express these molecules at physiological levels.
Fig. 6. Alternative coreceptor utilization by SIVsmm Envs in vitro. 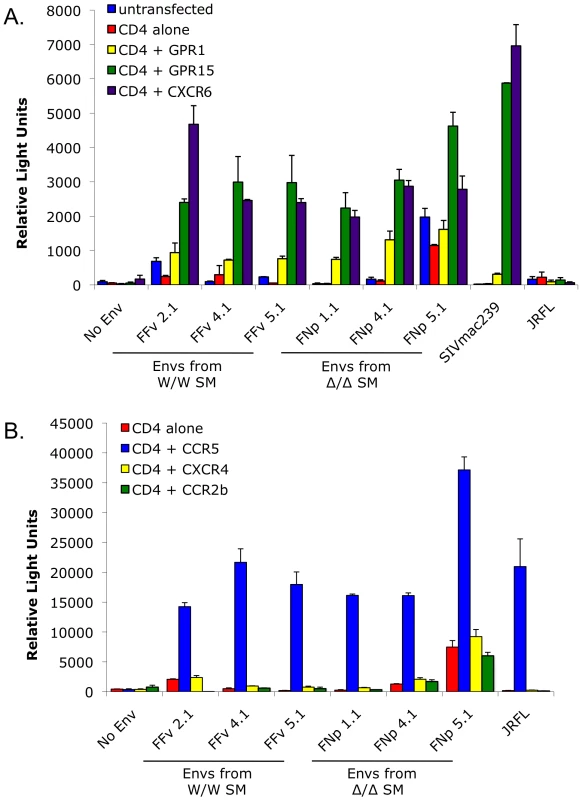
293T cells were transfected with CD4 alone or in combination with (A) the alternative coreceptors GPR1, GPR15 and CXCR6, or (B) CXCR4, CCR2b or CCR5. Target cells were then infected with luciferase-expressing pseudotype virions containing SIVsmm Envs from infected CCR5 wild-type (FFv: W/W SM) and CCR5-null (FNp: Δ2/Δ2 SM) animals. Envs from SIVmac239, HIV-1 JRFL and virions lacking Env served as controls. Infection was assayed by RLU (mean ± SD) measured 3 days after infection. SIVsmm infects sooty mangabey CCR5-null primary PBMC in vitro and infection of wild-type PBMC is not blocked by the CCR5 antagonist maraviroc
We next investigated the role of non-CCR5 pathways in SIVsmm infection of primary SM cells, utilizing both the specific CCR5 antagonist maraviroc and CCR5-null PBMC derived from CCR5Δ2 homozygous animals. Maraviroc blocks chemokine signaling and HIV-1 Env entry through human and rhesus macaque CCR5 [48], [49], but blocking of SM CCR5 coreceptor function has not been reported. Therefore, we first tested the effect of maraviroc on SIVsmm entry through smCCR5 in transfected cells. As shown in Figure 7A, maraviroc blocked SIVsmm pseudotype infection of target cells expressing CD4 and smCCR5, reducing luciferase expression to the level seen with target cells expressing CD4 alone (data not shown), indicating complete blocking of smCCR5-mediated entry by maraviroc. In contrast, maraviroc did not inhibit SIVsmm entry mediated by GPR15 (Figure 7A), nor did it affect entry by VSV-G pseudotypes (data not shown), confirming that blocking is not a nonspecific effect.
Fig. 7. Effect of CCR5 blocking on SIVsmm use of CCR5 and entry into primary SM PBMC. 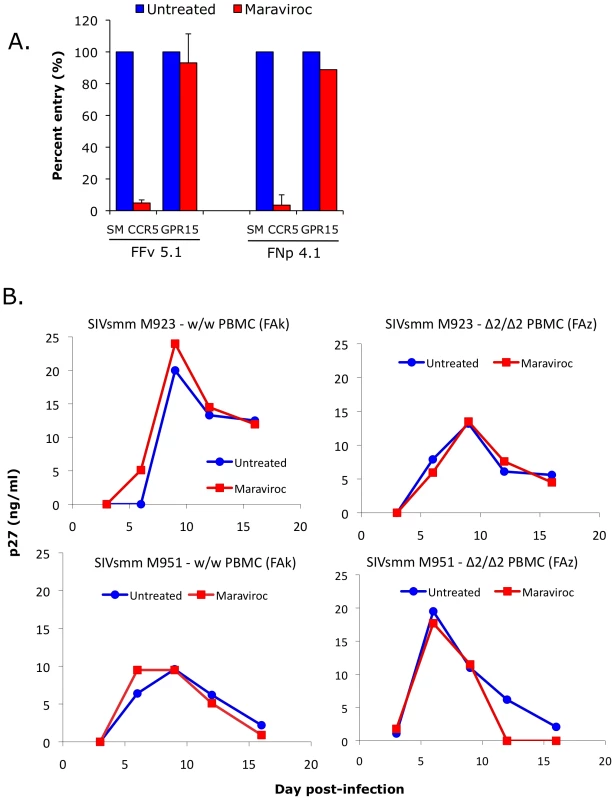
(A) 293T cells were transfected with CD4 in combination with wild-type smCCR5 or GPR15. Two days post-transfection, target cells were pretreated for one hour with or without the CCR5 antagonist, maraviroc (15uM), and then infected with pseudotype virions carrying SIVsmm Envs from a CCR5 wild-type animal (FFv) and a CCR5-null animal (FNp). Three days later, infection was measured based on RLU (mean ± SD) in cell lysates. (B) Growth curves from infection of primary SM PBMC (FAk: W/W SM; FAz: Δ2/Δ2 SM). Cells were stimulated for 3 days with PHA, then pretreated for one hour with or without maraviroc (15 uM), followed by infection with two different SIVsmm primary isolates (M923 and M951) in the continued presence or absence of maraviroc. SIV Gag p27 antigen levels in viral supernatants were measured by ELISA. Next we asked if maraviroc would affect productive infection of SM primary PBMC by infectious isolates of SIVsmm. PBMC from an uninfected CCR5 wild-type (FAk) and a CCR5-null (FAz) animals were activated with PHA for three days, and then infected in the presence and absence of maraviroc with two SIVsmm primary isolates (M923 and M951), which were both derived from CCR5 wild-type infected animals. Viral replication was measured as SIV gag p27 levels in supernatants collected periodically post-infection (Figure 7B).
We first noted that both SIVsmm isolates were able to productively infect primary PBMC in vitro from the CCR5-null SM (FAz). In these cells, there was no difference in replication associated with CCR5 blocking by maraviroc, as expected given the lack of functional CCR5 encoded by the mutant genes. This result indicates that SIVsmm infection of CCR5-null PBMC can occur independently of CCR5. We then tested CCR5 wild-type PBMC (FAk), and found that SIVsmm established productive infection in both the absence and presence of maraviroc. Furthermore, there was no difference in the level of infection achieved when CCR5 was blocked, based on p27 antigen production. This finding indicates that SIVsmm efficiently enters even CCR5-expressing SM primary PBMC through pathways independent of CCR5.
CCR5 genotypes in sooty mangabeys with SIV evolution to X4 coreceptor use
Unlike HIV-1 in humans, CXCR4 use by SIV in SM is rare. However, a few exceptions have been noted in which CXCR4 use emerged following experimental infection, which was associated with profound CD4+ T cell loss although not clinical AIDS [47]. Therefore, to ask if restricted CCR5+ target cell availability due to genetic absence of the coreceptor might be linked to CXCR4 emergence, we genotyped two infected CD4-low SM previously described in which CXCR4-using SIVsmm variants emerged [47]. Neither animal possessed a CCR5-null genotype: one was CCR5 homozygous wild type and the other was heterozygous for the CCR5Δ2 allele. Thus, the fact that acquisition of CXCR4 use by SIVsmm can occur but is not associated with animals that genetically lack CCR5 is consistent with the notion that alternative pathway-supported entry in vivo is robust and lack of CCR5 does not serve as a driving force in the rare cases with emergence of CXCR4 use.
Discussion
We unexpectedly found that 8% of sooty mangabeys in a large US captive population lack functional CCR5 due to the high prevalence (29%) of mutations in the CCR5 gene that abrogate cell surface expression and SIV coreceptor function. Despite their CCR5-null status, homozygous mutant animals are susceptible to natural as well as experimental SIVsmm infection and display viral loads only modestly lower than CCR5 wild-type animals. In vitro, SIVsmm enters primary SM lymphocytes independently of CCR5, and Envs from both wild type and CCR5-null infected animals use several alternative coreceptors in addition to CCR5, but do not use CXCR4. These data indicate that both CCR5 and alternative coreceptor pathways mediate cell entry and robust viral replication in vivo. The recognition that both CCR5-dependent and independent pathways are used in the SM natural host has significant implications for understanding viral tropism in vivo and CD4+ T cell subset targeting that may regulate the outcome of natural host infection, and raises important questions about entry coreceptor use in pathogenic non-human primate models of AIDS. This finding also explains the previously obscure reason for widespread and efficient use of alternative coreceptors among the SIVmac/smm family of viruses. Finally, it provides a third example of convergent evolution resulting in disruption of CCR5 function among primates, with consequences for virus-host interactions in SM that differ from both humans homozygous for CCR5Δ32 and red capped mangabeys homozygous for CCR5Δ24.
From the original descriptions of alternative coreceptor use by SIVmac/smm viruses, and subsequently by other SIV strains, the reason for conserved use of these pathways has remained elusive [20], [21], [50]. It has been repeatedly shown that various SIV isolates can infect primary human Δ32 homozygous PBMC independent of both CCR5 and CXCR4, indicating that alternative coreceptors are expressed in a manner that supports infection in primary lymphocytes ex vivo, at least in cells of human origin [51], [52], [53], [54], [55], [56]. Our data show for the first time that the alternative coreceptors are used by SIVsmm in primary simian T cells ex vivo and, more importantly, in vivo in the natural host from which the SIVmac/smm family derived. Several important questions are raised by this finding: (1) are alternative pathways also operative in SM with wild-type CCR5 expression, or are they only relevant if CCR5 is absent; (2) what essential role or selective advantage do they provide SIVsmm in SM infection in vivo that has led to conservation of alternative coreceptor entry pathway use; (3) does alternative coreceptor use define a novel population of CCR5-negative target cells that contributes to the ability of host and virus to coexist without disease, and; (4) what role do alternative pathways play in infection of macaques, the nonhuman primate model used to study AIDS.
Prior to this confirmation that alternative pathways are used in vivo, it seemed plausible that alternative coreceptor use by SIVsmm/mac was an in vitro epiphenomenon of little biological significance. Recognizing that they are operative in vivo, it seems more likely that conservation of their use among SIV isolates reflects some role that, if not completely essential, offers a selective advantage for the virus. Comparison of viral load data among the genotype groups showed robust replication in the absence of CCR5, but a step-wise increase associated with the presence of one or two functional CCR5 alleles, and ex vivo blocking studies with maraviroc confirmed that both wild-type and CCR5-null primary PBMC possess efficient CCR5-independent entry pathways. On one hand, if the relevant coreceptor(s) are expressed only on cells that also express CCR5, the use of multiple pathways in vivo might be an example of functional redundancy acquired by SIV, reminiscent in part of the functional redundancy of the chemokine/chemokine receptor system. On the other hand, if CCR5 and alternative pathways are expressed on distinct or only partially overlapping CD4+ T cell subsets, the 0.5 log10 VL difference between Δ/Δ and W/W animals may also be consistent with separate components of plasma viremia supported by CCR5 and non-CCR5 pathways (and an intermediate gene-dosage effect in the presence of one CCR5 allele). It is notable that the degree of depletion in SM gut CD4+ T cells exceeds the proportion that express detectable CCR5 [24], and it will be important to determine if this is due to infection and targeting of CCR5-negative cells in wild-type animals mediated by other coreceptors.
As to why alternative coreceptor use is conserved among SIVsmm and related strains, it seems unlikely to result from the 8% prevalence of CCR5-null animals in SM, and more likely reflects a unique role that provides an advantage over CCR5 alone in transmission, establishment of reservoirs or other aspects of infection. Sooty mangabeys overall express very low levels of CCR5 on CD4+ T cells, which has been proposed as an evolutionary adaptive response to “protect” critical target cells and minimize pathogenesis [17], [18]. If so, acquisition of alternative coreceptor use by the virus may have reflected a “counter-measure” to maximize replication capacity, although doing so in a manner that still retains the nonpathogenic nature of infection. Thus, the use of alternative entry pathways in vivo might enable infection of a novel CCR5-negative target cell population that is more expendable than CCR5+ cells, allowing the virus to replicate efficiently without causing disease in the face of extremely restricted CCR5 expression. It will therefore be important to define the distribution of cells infected in animals with and without functional CCR5.
An important question raised by these SM findings is whether alternative coreceptors are utilized in pathogenic infection of macaques, which is widely used to model human AIDS. While CCR5 clearly plays a principal role as evidenced by substantial albeit variable viral suppression by CCR5 antagonists [57], [58], levels of CD4+ T cell infection in rhesus macaques can also substantially exceed the proportion of cells that express detectable CCR5 [23]. While it is possible that infection of apparently CCR5-negative targets reflects entry mediated by CCR5 at levels below the threshold detectable by FACS, our findings revive the question of whether it may be mediated by additional entry pathways. Few studies have attempted to address alternative coreceptor use in vivo. One report showed that mutations that abrogated GPR15 use by SIVmac had little effect on replication or pathogenesis in rhesus macaques [22]. Similarly, when pigtail macaques (Macaca nemestrina) were infected with SIVmne (also derived from SIVsmm), serial isolates exhibited decreasing ability to use alternative entry pathways when assayed in vitro [51]. On the other hand, in cynomolgus macaques (Macaca fascicularis) infected with SIVsmm, animals with progressive disease showed retention or broadening of alternative coreceptor use while those without disease progression showed narrowing of alternative coreceptor use [52]. Thus, it remains to be determined whether alternative pathways support entry in particular subsets of CD4+ T cells in the macaque model in vivo. Of note, emergence of CXCR4 use is common in HIV-1 infection of humans but exceedingly infrequent in macaques infected with SIVmac. HIV-1 rarely uses alternative coreceptors efficiently and evolution to CXCR4 use by HIV-1 is believed to result, in part, from loss of CCR5+ target cells in late stage disease. Thus, the availability of efficient alternative entry pathways may be one reason that SIV rarely evolves to use CXCR4.
The SIVsmm Envs examined here use GPR15 and CXCR6 for entry quite efficiently in vitro, and use GPR1 somewhat less efficiently. This result is concordant with coreceptor use patterns of multiple other SIV isolates [22], [23], [30]. In human blood cells, CXCR6 is highly expressed on CD4+ and CD8+ memory but not naïve T cells, and on gamma-delta T and to a lesser extent NK cells [59], although others have reported expression by CD4+ naïve T cells as well [60]. Interestingly, CXCR6 expression is regulated by T cell activation in a pattern that is tightly linked with CCR5 expression in response to some stimuli, but markedly different in response to others [59]. GPR15 is also expressed on lymphoid and myeloid cells but with less information about specific distribution patterns [20], [21], [61]. Of note, both CXCR6 and GPR15 are also highly expressed in intestinal tissues [20], [62], raising the question of whether alternative coreceptor use may be involved in mucosal events that play a central role in infection. Thus, CXCR6 and GPR15 are particularly likely candidates for mediating SM infection independent of CCR5, and further studies are required to determine which one or ones are responsible for entry into primary SM PBMC ex vivo and infection in vivo.
One of the most important priorities in HIV/AIDS research at present is understanding why infected natural hosts remain healthy while rhesus macaques infected with SIV, humans infected with HIV-1 or HIV-2, and chimpanzees infected with SIVcpz develop AIDS. Many features are shared by non-pathogenic natural host and pathogenic non-natural host infection including sustained high level viremia, vigorous immune activation during acute infection, and extensive depletion of gut mucosal CD4+ T cells. In pathogenic infections it is believed that in addition to infection and loss of short-lived T effector and T effector memory cells, damage to long-lived CD4+ Tcm populations that impairs the capacity to maintain immune cell homeostasis is a critical factor in progressive immunodeficiency [26], [27], [63]. CCR5 levels are profoundly lower on SM CD4+ T cells compared with RM and humans, which may restrict the target cells available for infection in vivo [17], [18]. More recent findings indicate that CD4+ Tcm in SM have particularly impaired CCR5 expression upon activation, and this corresponds with markedly lower levels of cell-associated infection in Tcm compared with Tem cells in SM, whereas both populations are similarly infected in RM (G.S. and M.P; unpublished observations). Thus, in addition to the role of CCR5, it will be important to define the distribution and use of other coreceptors by SIVsmm in its natural host as well as in pathogenic rhesus macaque infection. Another prominent difference between pathogenic infection and nonpathogenic natural host infection is the presence of sustained high level generalized immune activation in rhesus and humans, whereas acute infection in natural hosts is associated with transient immune activation that rapidly resolves [11], [29], [35], [64], [65], [66]. Sustained immune activation during chronic infection may be an additional factor driving T cell turnover and depletion. A principal mechanism driving chronic immune activation is believed to be translocation of microbial products due to disruption of gut mucosal barrier integrity that occurs early in infection [31]. However, gut mucosal lymphocyte infection and CD4+ T cell depletion occurs in natural host as well as pathogenic host infection [24], [33]. One potentially critical difference is the loss in human and rhesus macaque infection, but preservation in natural hosts, of mucosal CD4 Th17 cells, which play a critical role in gut mucosal immune defense [35], [36], [37], [38]. Why gut Th17 CD4+ T cells are preserved in infected natural hosts but depleted in other hosts remains to be determined, but the critical role of entry coreceptors in determining tropism and cell subset infection in vivo suggest that both CCR5 and alternative coreceptor pathways must be defined in order to understand the targeting versus protection of critical CD4+ cell subsets.
In addition to the YNPRC and TNPRC SM colonies, we also found the Δ2 allele in SM in the Tai forest of Cote d'Ivoire, confirming its presence not just in captive but also in wild-living West African animals, albeit at a lower frequency. Sooty mangabeys at YNPRC were derived from multiple sources and their history is not well documented [67], so it is difficult to know for sure from what geographic regions in West Africa these animals descended. Of note, the Δ2 allele was not described in earlier studies that reported the Δ24 allele in SM from West Africa and the YNPRC colony [43], [44]. However, CCR5 alleles in those studies were screened by PCR amplicon size, which could discriminate a 24 bp size difference but is unlikely to distinguish a 2 bp difference between the wild type and Δ2 alleles.
The identification here of SM CCR5Δ2 brings to three the number of primates known to have a high prevalence of defective CCR5 alleles. Interestingly, each of the three examples of populations with homozygous mutant CCR5 individuals shows distinct patterns of host/virus interactions. For HIV-1 in humans, which have the lowest prevalence of defective CCR5 genes (∼1% among Caucasians), CCR5 use is a stringent requirement for establishment of new infections, and its absence in the host provides almost complete protection from infection even though late-stage variants can use CXCR4 and, occasionally, other coreceptors. In RCM, which have the highest prevalence of CCR5-null individuals (≥70%), SIVrcm has adapted to use CCR2b as a coreceptor and lost the ability to use CCR5 [43]. SIVsmm infection in SM, which have an intermediate prevalence of CCR5-null individuals (8% in this population), demonstrate an intermediate relationship, in which alternative coreceptors efficiently mediate both natural and experimental infection in the absence of CCR5, but CCR5 use by the virus is retained, perhaps because both CCR5 and alternative pathways together maximize replication in the majority of animals, while the non-CCR5 pathways are required in the CCR5-null animals.
It is somewhat unexpected that SIVsmm does not use CCR2b, which is the route of entry taken by SIVrcm in the absence of CCR5. SM and RCM are closely related and sometimes considered sub-species within the same species [43], [68]. The presence of the same CCR5Δ24 allele in RCM and SM has been interpreted as indicating an origin prior to separation of these populations, although it is uncertain if the remarkably high frequency CCR5Δ24 in the RCM population reflects selective pressure exerted by an environmental or infectious cause, or founder effect [43]. Another question raised by our results is whether the SM CCR5Δ2 and the SM/RCM CCR5Δ24 emerged independently, resulting in deletions in the same region due to multiple nucleotide repeats enabling recombination, or given the overlapping sites whether Δ2 emerged first and additional events led to Δ24. Since Δ2 is not expressed, it is unlikely that further deletions would lead to any additional selective advantage, and two separate recombination deletion events in the same region of the same gene seem more probable.
What selective pressures might have led to three independent primate CCR5 deletion alleles is uncertain. CCR5Δ32 has been present in the human population for at least 3000 years [69], far longer than HIV-1, and despite considerable speculation on infectious or other pressures, both what factors fueled its emergence and when it occurred remain enigmatic [70]. In contrast, SIV has been endemic in the SM and RCM populations for much longer, although whether its entry predated separation of the populations is uncertain [68]. The CCR5 mutation is currently not essential for protection from SIV-induced pathogenesis, but it is plausible that each of the mutations result from ancestral evolutionary pressure by pathogenic SIV infection. For SM, genetic abrogation of CCR5 expression may have been an additional, complementary response to control pathogenesis along with phenotypic CCR5 downregulation [18]. If so, in both SM and RCM the virus then acquired mechanisms to circumvent the restriction, by expanding coreceptor use for SIVsmm or switching for SIVrcm, yet the hosts then acquired other additional mechanisms to avoid pathogenesis. Alternatively, it may be that all three CCR5 mutations, human, SM and RCM, reflect evolutionary adaptation to some other as-yet unidentified infectious or other environmental factor acting similarly on all three types of primates. Nevertheless, whatever its origin and frequency, the SM CCR5Δ2 mutation here expands the pathways known to support infection in this important natural host model, the identity, distribution and utilization of which must be taken into account in understanding SM infection in vivo, and the similarities or differences from RM infection that determine outcome from infection.
Materials and Methods
Ethics statement
All animal experimentation was conducted following guidelines established by the Animal Welfare Act and the NIH for housing and care of laboratory animals and performed in accordance with Institutional regulations after review and approval by the Institutional Animal Care and Use Committees (IACUC) at the Yerkes National Primate Research Center (YNPRC) or the Tulane National Primate Research Center (TNPRC). Studies were also reviewed and approved by the University of Pennsylvania IACUC.
Animals and primary cells
These studies utilized blood cells from animals housed at the YNPRC or TNPRC. Peripheral blood mononuclear cells (PBMC) were isolated from whole blood using standard density gradient separation methods. For genomic analysis, approximately 0.8×106 PBMC were lysed in DNA lysis buffer (100 mM KCl; 0.1% NP40; 20 mM Tris pH 8.4; 0.5 mg/ml proteinase K; 200ul total volume) and used as a template for PCR amplification. For infection studies, cryopreserved PBMC were thawed under standard conditions and maintained at 106 cells/ml in RPMI supplemented with 10% FBS, 1% glutamine and 1% penicillin-streptomycin, stimulated for 3 days with 5 µg/ml of phytohemagglutinin (PHA; MP Biomedical), infected and then maintained in the same media in the presence of IL-2 (50 U/ml; Novartis). Analysis of wild SM genotypes was carried out on purified DNA derived from fecal specimens collected in Cote d'Ivoire, which were previously characterized by mitochondrial DNA sequence analysis to represent 33 distinct individuals [45].
Cloning SM CCR5 genes
Full-length SM-CCR5 genes were amplified by PCR from SM genomic DNA using high fidelity DNA polymerase (Phusion; Finnzymes) and primers based on conserved 5′ and 3′ regions of published SM CCR5 coding sequences (forward: 5′-ATG GAC TAT CAA GTG TCA AGT CCA ACC-3′; reverse: 5′-TCA CAA GCC AAC AGA TAT TTC CTG CTC C-3′). PCR reactions contained Phusion polymerase (1 unit) in HF buffer, primers (0.5 uM), dNTPs (0.2 mM) and 200 ng of purified genomic DNA as template in a 50 ul reaction volume. Thermocycling conditions: initial denaturation at 98°C for 45 seconds, followed by 20 cycles of 98°C for 10 seconds, 71°C for 30 seconds and 72°C for 90 seconds, with a final extension step of 72°C for 10 minutes. PCR amplicons were column purified (QIAquick PCR Purification kit; Qiagen) and then used in a second PCR reaction employing primers that incorporated a HindIII restriction site at the 5′ end of the coding region and a BamHI restriction site at the 3′ end of the CCR5 coding sequence (forward: 5′-GCT GCT ATA AGC TTC CAC CAT GGA CTA TCA AG-3′; reverse: 5′-AGC GAG CGG ATC CTC ACA AGC CAA CAG ATA-3′; restriction sites underlined). Thermocycling conditions for the second PCR reaction were: an initial denaturation step at 98°C for 45 seconds, followed by 5 cycles of 98°C for 10 seconds, 71°C for 45 seconds and 72°C for 60 seconds, followed by 15 cycles of 98°C for 10 seconds, 76°C for 45 seconds and 72°C for 60 seconds, and final extension at 72°C for 10 minutes. CCR5 amplicons were cloned into the expression plasmid pcDNA3.1+ (Invitrogen) using HindIII and BamHI, and screened by restriction analysis followed by sequence confirmation. Sequences of the smCCR5Δ2, smCCR5Δ24 and smCCR5 wild-type genes cloned here have been deposited in Genbank (accession numbers HM246694, HM246695 and HM246693, respectively).
CCR5 genotyping of SM
A two-step PCR-based genotyping assay was developed that identifies CCR5 wild-type and CCR5Δ2 alleles based on differential primer annealing, using genomic DNA from lysates of SM PBMC. Genomic DNA was subject to PCR amplification using the first round primers described above, to amplify the entire CCR5 coding region, in reactions that contained Platinum Taq polymerase (1 unit; Invitrogen), 1.5 mM MgCl2, primers (0.5 uM each), dNTPs (0.2 mM), 1–3 ul DNA lysate and Taq buffer in 25 ul reaction volumes. Thermocycling conditions were: initial denaturation at 98°C for 45 second followed by 10 cycles of 94°C for 20 seconds, 68°C for 45 seconds, 72°C for 60 seconds and final extension at 72°C for 10 minutes. The product of this reaction (2.5 ul) was then used as a template for two separate second-round amplifications, each of which used a common downstream primer but different upstream primers specific for the wild-type and Δ2 alleles, respectively (forward CCR5 wild-type: 5′-ATC ACT TGG GTG GTG GCT-3′; forward CCR5Δ2 : 5′-ATC ACT TGG GTG GTG CGT-3′; common downstream: 5′-GGT GTT CAG GAG AAG GAC AAT GTT G-3′). The second round PCR reaction used the same conditions as the first reaction. Products of the wild-type and Δ2 amplification reactions were visualized by 2% agarose gel electrophoresis and ethidium bromide staining, which demonstrated a 325 base pair product following amplification with wild-type or Δ2 primer pairs, or both for heterozygotes (Figure S1A).
Each animal was also screened for the CCR5Δ24 deletion allele with a two-step PCR-based assay. Products of the first round PCR reaction described above, were subjected to nested amplification with inner primers (forward: 5′-GGC TAT CGT CCA TGC TGT GT-3′; reverse: 5′-GAC CAG CCC CAA GAT GAC TA-3′) and thermocycling conditions as follows: initial denaturation at 94°C for 45 seconds, followed by 25 cycles of 94°C for 20 seconds, 59°C for 45 seconds, 72°C for 60 second, and a final extension at 72°C for 10 minutes. Products were visualized by 3% agarose gel electrophoresis and ethidium bromide staining, yielding a 205 base pair product from the Δ24 allele, which was easily distinguishable from the 227–229 bp product from the wild-type and CCR5 Δ2 alleles (Figure S1B).
As a secondary confirmation of genotypes established by PCR screening, direct bulk sequencing was carried out on PCR amplified genomic DNA. Amplification was done using the outer primer set as described above, except that Phusion high fidelity polymerase was used for 30 cycles. Products were column-purified and sequenced. Genotypes were verified by manual inspection to confirm the presence of uniform sequences for homozygous animals, or detection of expected frameshifts resulting in overlapping sequences for heterozygous animals (Figure S1C).
For fecal-derived samples, 0.5 ug of purified DNA (of which only a fraction reflected host-derived DNA) was PCR amplified for 35 cycles using first round outer primers as described above, and then subjected to nested amplification for 30 cycles using the inner primer set described above. Products were then subjected to direct sequence analysis.
Pseudotype luciferase reporter virus infections and coreceptor use analysis
SIVsmm Env-mediated entry was analyzed using luciferase-expressing reporter viruses pseudotyped with envelope glycoproteins of interest. Pseudotype viruses were generated by co-transfecting 293T cells with a plasmid encoding the NL4-3-based env-deleted luciferase-expressing virus backbone (pNL-luc-E−R+) [71] along with expression plasmids encoding SIVsmm, SIVmac, HIV-1 or VSV-g envelope glycoproteins. Cells were transfected overnight using Fugene (Roche) and washed the next day to remove residual transfection reagent. Supernatants were collected 2 days later, clarified by centrifugation and stored at −80°C until use. Pseudotype viruses were quantified based on HIV-1 Gag p24 antigen ELISA (PerkinElmer) and virion infectivity measured on U87 cells stably expressing CD4 and CCR5. Inocula were then standardized on the basis of infectivity in U87/CD4/CCR5 cells (1×106 relative light units; RLU).
The ability of pseudotype viruses to use different coreceptors was assayed in target 293T cells expressing CD4 and the coreceptor of interest. Target cells were prepared by co-transfection with expression plasmids carrying CD4 and the desired co-receptor (1ug of each plasmid) using Fugene. Cells were re-plated one day post-transfection at 2×104 cells/well in 96-well plates and then infected the following day with pseudotype reporter viruses using equivalent inocula (1×106 RLU) by spin inoculation for 2 hours at 1200G. Three days later cells were lysed (0.5% Triton X-100 in PBS) and infection quantified on the basis of luciferase production in target cells, determined by adding an equal volume of luciferase substrate (Promega) and measuring luciferase activity in RLU with a luminometer.
SIV envelope clones and infectious viruses
SIV envelopes used in pseudotype infections were generated from plasma of SIVsmm-infected CCR5 wild-type (FFv) and Δ2 homozygous (FNp) SM by single genome amplification (SGA) using methods and protocols previously described and cloned into pcDNA3.1 using Topo TA (Invitrogen) [72], [73]. Infectious SIVsmm strains M923 and M951 were isolated as previously described [74] and stocks were prepared in primary SM PBMC.
FACS analysis of wild-type and mutant CCR5 surface expression
293T cells were transfected with wild-type or mutant forms of CCR5 using Fugene according to manufacturer's instructions. One day later cells were detached by incubation in PBS containing 2 mM EDTA, washed in FACS buffer (PBS containing 1% FBS and 0.1% sodium azide), and stained with the CCR5 monoclonal antibody, clone 3A9-[APC] (BD Pharmingen) or isotype-matched control. Cells were analyzed using a FACS Caliber flow cytometer (BD Biosciences) and FloJo software (Tree Star, Inc.) to determine CCR5 surface expression.
SIV infections of primary SM PBMC
PHA-stimulated SM PBMC were plated in 96-well plates at 2.5×105 cells/well, incubated for 1 hour in the presence or absence of the CCR5 antagonist maraviroc (15 uM; Pfizer), and then infected with SIVsmm strains (M923 and M951) by spin inoculation (1200×g for 2 hours) followed by overnight incubation. The next day cells were washed in PBS and maintained in media containing IL-2 (50 U/ml), with or without maraviroc (15 uM). Cell supernatants were collected periodically for 3 weeks and replication measured by SIV Gag p27 antigen in cell supernatant by ELISA (Advanced BioScience Laboratories).
Analysis of SM virological and immunological parameters
Clinical data on infected and uninfected SM housed at YNPRC has been reported previously for surveys carried out in 2004–5 and 2006–7 and 2008–9 [75]. CD3, CD4 and CCR5 expression on PBMC were analyzed by FACS and plasma viral loads were measured by real-time PCR as described [11], [75]. For purposes of analysis, any undetectable viral loads were set at 75 copies, the lower limit of detection. In cases where animals seroconverted during the period of observation, data from prior to infection was included with uninfected animals, while data from after infection was included with infected animals. If multiple measurements were available for individual animals for any parameters, mean values were utilized.
Statistical analysis
Virological and immunological data were compared between genotype groups using ANOVA or Kruskal-Wallis test followed by the Dunn's multiple comparison test for multiple groups. The two-sample proportions test and Chi-Square test were used for comparison between independent groups. Statistical tests were performed using Prism 4.0 software and OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version 2.3. Data were considered significant when P-value was below 0.05.
Supporting Information
Zdroje
1. GaoF
BailesE
RobertsonDL
ChenY
RodenburgCM
1999 Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397 436 441
2. BailesE
GaoF
Bibollet-RucheF
CourgnaudV
PeetersM
2003 Hybrid origin of SIV in chimpanzees. Science 300 1713
3. PeetersM
JanssensW
FransenK
BrandfulJ
HeyndrickxL
1994 Isolation of simian immunodeficiency viruses from two sooty mangabeys in Cote d'Ivoire: virological and genetic characterization and relationship to other HIV type 2 and SIVsm/mac strains. AIDS Res Hum Retroviruses 10 1289 1294
4. HirschVM
OlmstedRA
Murphey-CorbM
PurcellRH
JohnsonPR
1989 An African primate lentivirus (SIVsm) closely related to HIV-2. Nature 339 389 392
5. ApetreiC
KaurA
LercheNW
MetzgerM
PandreaI
2005 Molecular epidemiology of simian immunodeficiency virus SIVsm in U.S. primate centers unravels the origin of SIVmac and SIVstm. J Virol 79 8991 9005
6. SilvestriG
PaiardiniM
PandreaI
LedermanMM
SodoraDL
2007 Understanding the benign nature of SIV infection in natural hosts. J Clin Invest 117 3148 3154
7. SodoraDL
AllanJS
ApetreiC
BrenchleyJM
DouekDC
2009 Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nat Med 15 861 865
8. KeeleBF
JonesJH
TerioKA
EstesJD
RudicellRS
2009 Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature 460 515 519
9. Rey-CuilleMA
BerthierJL
Bomsel-DemontoyMC
ChaducY
MontagnierL
1998 Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J Virol 72 3872 3886
10. BroussardSR
StapransSI
WhiteR
WhiteheadEM
FeinbergMB
2001 Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J Virol 75 2262 2275
11. SilvestriG
SodoraDL
KoupRA
PaiardiniM
O'NeilSP
2003 Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18 441 452
12. HolzammerS
HolznagelE
KaulA
KurthR
NorleyS
2001 High virus loads in naturally and experimentally SIVagm-infected African green monkeys. Virology 283 324 331
13. KootM
KeetIPM
RosAHV
de GoedeREY
RoosMTL
1993 Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. AnnInternMed 118 681 688
14. ScarlattiG
TresoldiE
BjorndalA
FredrikssonR
ColognesiC
1997 In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med 3 1259 1265
15. BlaakH
van't WoutAB
BrouwerM
HooibrinkB
HovenkampE
2000 In vivo HIV-1 infection of CD45RA+CD4+ T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4+ T cell decline. ProcNatlAcadSciUSA 97 1269 1274
16. Van RijRP
BlaakH
VisserJA
BrouwerM
RientsmaR
2000 Differential coreceptor expression allows for independent evolution of non-syncytium-inducing and syncytium-inducing HIV-1. JClinInvest 106 1039 1052
17. PandreaI
OnangaR
SouquiereS
Mouinga-OndemeA
BourryO
2008 Paucity of CD4+ CCR5+ T cells may prevent transmission of simian immunodeficiency virus in natural nonhuman primate hosts by breast-feeding. J Virol 82 5501 5509
18. PandreaI
ApetreiC
GordonS
BarbercheckJ
DufourJ
2007 Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood 109 1069 1076
19. RuckerJ
EdingerAL
SharronM
SamsonM
LeeB
1997 Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol 71 8999 9007
20. DengHK
UnutmazD
KewalRamaniVN
LittmanDR
1997 Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 388 296 300
21. FarzanM
ChoeH
MartinK
MarconL
HofmannW
1997 Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med 186 405 411
22. PohlmannS
StolteN
MunchJ
Ten HaaftP
HeeneyJL
1999 Co-receptor usage of BOB/GPR15 in addition to CCR5 has no significant effect on replication of simian immunodeficiency virus in vivo. J Infect Dis 180 1494 1502
23. MattapallilJJ
DouekDC
HillB
NishimuraY
MartinM
2005 Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434 1093 1097
24. GordonSN
KlattNR
BosingerSE
BrenchleyJM
MilushJM
2007 Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol 179 3026 3034
25. McCuneJM
2001 The dynamics of CD4+ T-cell depletion in HIV disease. Nature 410 974 979
26. GrossmanZ
Meier-SchellersheimM
PaulWE
PickerLJ
2006 Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med 12 289 295
27. OkoyeA
Meier-SchellersheimM
BrenchleyJM
HagenSI
WalkerJM
2007 Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med 204 2171 2185
28. LiuZ
CumberlandWG
HultinLE
PrinceHE
DetelsR
1997 Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol 16 83 92
29. BosingerSE
LiQ
GordonSN
KlattNR
DuanL
2009 Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest 119 3556 3572
30. JacquelinB
MayauV
TargatB
LiovatAS
KunkelD
2009 Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest 119 3544 3555
31. BrenchleyJM
PriceDA
SchackerTW
AsherTE
SilvestriG
2006 Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12 1365 1371
32. BrenchleyJM
SchackerTW
RuffLE
PriceDA
TaylorJH
2004 CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 200 749 759
33. PandreaIV
GautamR
RibeiroRM
BrenchleyJM
ButlerIF
2007 Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol 179 3035 3046
34. VeazeyRS
DeMariaM
ChalifouxLV
ShvetzDE
PauleyDR
1998 Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280 427 431
35. FavreD
LedererS
KanwarB
MaZM
ProllS
2009 Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog 5 e1000295
36. RaffatelluM
SantosRL
VerhoevenDE
GeorgeMD
WilsonRP
2008 Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med 14 421 428
37. CecchinatoV
TrindadeCJ
LaurenceA
HeraudJM
BrenchleyJM
2008 Altered balance between Th17 and Th1 cells at mucosal sites predicts AIDS progression in simian immunodeficiency virus-infected macaques. Mucosal Immunol 1 279 288
38. BrenchleyJM
PaiardiniM
KnoxKS
AsherAI
CervasiB
2008 Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112 2826 2835
39. LiuR
PaxtonWA
ChoeS
CeradiniD
MartinSR
1996 Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86 367 377
40. SamsonM
LibertF
DoranzBJ
RuckerJ
LiesnardC
1996 Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382 722 725
41. HuangY
PaxtonW
WolinskySM
NeumannAU
ZhangL
1996 The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nature Med 2 1240 1243
42. DeanM
CarringtonM
WinklerC
HuttleyGA
SmithMW
1996 Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CCR5 structural gene. Science 273 1856 1862
43. ChenZ
KwonD
JinZ
MonardS
TelferP
1998 Natural infection of a homozygous delta24 CCR5 red-capped mangabey with an R2b-tropic simian immunodeficiency virus. J Exp Med 188 2057 2065
44. PalaciosE
DigilioL
McClureHM
ChenZ
MarxPA
1998 Parallel evolution of CCR5-null phenotypes in humans and in a natural host of simian immunodeficiency virus. Curr Biol 8 943 946
45. SantiagoML
RangeF
KeeleBF
LiY
BailesE
2005 Simian immunodeficiency virus infection in free-ranging sooty mangabeys (Cercocebus atys atys) from the Tai Forest, Cote d'Ivoire: implications for the origin of epidemic human immunodeficiency virus type 2. J Virol 79 12515 12527
46. ApetreiC
GautamR
SumpterB
CarterAC
GaufinT
2007 Virus subtype-specific features of natural simian immunodeficiency virus SIVsmm infection in sooty mangabeys. J Virol 81 7913 7923
47. MilushJM
ReevesJD
GordonSN
ZhouD
MuthukumarA
2007 Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J Immunol 179 3047 3056
48. SaitaY
KondoM
ShimizuY
2007 Species selectivity of small-molecular antagonists for the CCR5 chemokine receptor. Int Immunopharmacol 7 1528 1534
49. KetasTJ
KuhmannSE
PalmerA
ZuritaJ
HeW
2007 Cell surface expression of CCR5 and other host factors influence the inhibition of HIV-1 infection of human lymphocytes by CCR5 ligands. Virology 364 281 290
50. AlkhatibG
LiaoF
BergerEA
FarberJM
PedenKW
1997 A new SIV co-receptor, STRL33. Nature 388 238
51. ForteS
HarmonME
PinedaMJ
OverbaughJ
2003 Early - and intermediate-stage variants of simian immunodeficiency virus replicate efficiently in cells lacking CCR5. J Virol 77 9723 9727
52. LaurenA
VodrosD
ThorstenssonR
FenyoEM
2006 Comparative studies on mucosal and intravenous transmission of simian immunodeficiency virus (SIVsm): evolution of coreceptor use varies with pathogenic outcome. J Gen Virol 87 581 594
53. ChenZ
ZhouP
HoDD
LandauNR
MarxPA
1997 Genetically divergent strains of simian immunodeficiency virus use CCR5 as a coreceptor for entry. J Virol 71 2705 2714
54. ZhangY
LouB
LalRB
GettieA
MarxPA
2000 Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells. J Virol 74 6893 6910
55. ChenZ
GettieA
HoDD
MarxPA
1998 Primary SIVsm isolates use the CCR5 coreceptor from sooty mangabeys naturally infected in west Africa: a comparison of coreceptor usage of primary SIVsm, HIV-2, and SIVmac. Virology 246 113 124
56. OwenSM
MasciotraS
NovembreF
YeeJ
SwitzerWM
2000 Simian immunodeficiency viruses of diverse origin can use CXCR4 as a coreceptor for entry into human cells. J Virol 74 5702 5708
57. WolinskySM
VeazeyRS
KunstmanKJ
KlassePJ
DufourJ
2004 Effect of a CCR5 inhibitor on viral loads in macaques dual-infected with R5 and X4 primate immunodeficiency viruses. Virology 328 19 29
58. VeazeyRS
KlassePJ
KetasTJ
ReevesJD
PiatakMJr
2003 Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J Exp Med 198 1551 1562
59. UnutmazD
XiangW
SunshineMJ
CampbellJ
ButcherE
2000 The primate lentiviral receptor Bonzo/STRL33 is coordinately regulated with CCR5 and its expression pattern is conserved between human and mouse. J Immunol 165 3284 3292
60. SharronM
PohlmannS
PriceK
LolisE
TsangM
2000 Expression and coreceptor activity of STRL33/Bonzo on primary peripheral blood lymphocytes. Blood 96 41 49
61. ElbimC
MonceauxV
MuellerYM
LewisMG
FrancoisS
2008 Early divergence in neutrophil apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J Immunol 181 8613 8623
62. LiQ
EstesJD
DuanL
JessurunJ
PambuccianS
2008 Simian immunodeficiency virus-induced intestinal cell apoptosis is the underlying mechanism of the regenerative enteropathy of early infection. J Infect Dis 197 420 429
63. PickerLJ
HagenSI
LumR
Reed-InderbitzinEF
DalyLM
2004 Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J Exp Med 200 1299 1314
64. GiorgiJV
HultinLE
McKeatingJA
JohnsonTD
OwensB
1999 Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis 179 859 870
65. EstesJD
GordonSN
ZengM
ChahroudiAM
DunhamRM
2008 Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol 180 6798 6807
66. LedererS
FavreD
WaltersKA
ProllS
KanwarB
2009 Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog 5 e1000296
67. BernsteinI
1971 The influence of introductory techniques on the formation of captive mangabey groups. Primates 12 33 44
68. Georges-CourbotMC
LuCY
MakuwaM
TelferP
OnangaR
1998 Natural infection of a household pet red-capped mangabey (Cercocebus torquatus torquatus) with a new simian immunodeficiency virus. J Virol 72 600 608
69. HummelS
SchmidtD
KremeyerB
HerrmannB
OppermannM
2005 Detection of the CCR5-Delta32 HIV resistance gene in Bronze Age skeletons. Genes Immun 6 371 374
70. HedrickPW
VerrelliBC
2006 “Ground truth” for selection on CCR5-Delta32. Trends Genet 22 293 296
71. ConnorRI
ChenBK
ChoeS
LandauNR
1995 Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206 935 944
72. RongR
LiB
LynchRM
HaalandRE
MurphyMK
2009 Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog 5 e1000594
73. BurioniR
PlaisantP
RiccioML
RossoliniGM
SantangeloR
1995 Engineering human monoclonal antibody fragments: a recombinant enzyme-linked Fab. New Microbiol 18 127 133
74. GautamR
CarterAC
KatzN
ButlerIF
BarnesM
2007 In vitro characterization of primary SIVsmm isolates belonging to different lineages. In vitro growth on rhesus macaque cells is not predictive for in vivo replication in rhesus macaques. Virology 362 257 270
75. TaaffeJ
ChahroudiA
EngramJ
SumpterB
MeekerT
A five-year longitudinal analysis of naturally SIV-infected sooty mangabeys reveals a slow but progressive CD4+ T-cell decline whose magnitude is not predicted by viral load or immune activation. J Virol
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 8- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Dissecting the Genetic Architecture of Host–Pathogen Specificity
- The Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts
- Global Genotype-Phenotype Correlations in
- Burkholderia Type VI Secretion Systems Have Distinct Roles in Eukaryotic and Bacterial Cell Interactions
- Chitin Synthases from Are Involved in Tip Growth and Represent a Potential Target for Anti-Oomycete Drugs
- Distinct Merkel Cell Polyomavirus Molecular Features in Tumour and Non Tumour Specimens from Patients with Merkel Cell Carcinoma
- Biological and Structural Characterization of a Host-Adapting Amino Acid in Influenza Virus
- Functional Characterisation and Drug Target Validation of a Mitotic Kinesin-13 in
- CTCF Prevents the Epigenetic Drift of EBV Latency Promoter Qp
- The Human Fungal Pathogen Escapes Macrophages by a Phagosome Emptying Mechanism That Is Inhibited by Arp2/3 Complex-Mediated Actin Polymerisation
- Bim Nuclear Translocation and Inactivation by Viral Interferon Regulatory Factor
- Cyst Wall Protein 1 Is a Lectin That Binds to Curled Fibrils of the GalNAc Homopolymer
- Reciprocal Analysis of Infections of a Model Reveal Host-Pathogen Conflicts Mediated by Reactive Oxygen and imd-Regulated Innate Immune Response
- A Subset of Replication Proteins Enhances Origin Recognition and Lytic Replication by the Epstein-Barr Virus ZEBRA Protein
- Damaged Intestinal Epithelial Integrity Linked to Microbial Translocation in Pathogenic Simian Immunodeficiency Virus Infections
- Kaposin-B Enhances the PROX1 mRNA Stability during Lymphatic Reprogramming of Vascular Endothelial Cells by Kaposi's Sarcoma Herpes Virus
- Direct Interaction between Two Viral Proteins, the Nonstructural Protein 2C and the Capsid Protein VP3, Is Required for Enterovirus Morphogenesis
- A Novel CCR5 Mutation Common in Sooty Mangabeys Reveals SIVsmm Infection of CCR5-Null Natural Hosts and Efficient Alternative Coreceptor Use
- Micro RNAs of Epstein-Barr Virus Promote Cell Cycle Progression and Prevent Apoptosis of Primary Human B Cells
- Enterohemorrhagic Requires N-WASP for Efficient Type III Translocation but Not for EspF-Mediated Actin Pedestal Formation
- Host Imprints on Bacterial Genomes—Rapid, Divergent Evolution in Individual Patients
- UNC93B1 Mediates Host Resistance to Infection with
- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- Protective Efficacy of Cross-Reactive CD8 T Cells Recognising Mutant Viral Epitopes Depends on Peptide-MHC-I Structural Interactions and T Cell Activation Threshold
- Bacteriophage Lysin Mediates the Binding of to Human Platelets through Interaction with Fibrinogen
- Insecticide Control of Vector-Borne Diseases: When Is Insecticide Resistance a Problem?
- Immune Modulation with Sulfasalazine Attenuates Immunopathogenesis but Enhances Macrophage-Mediated Fungal Clearance during Pneumonia
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- A Multi-Step Process of Viral Adaptation to a Mutagenic Nucleoside Analogue by Modulation of Transition Types Leads to Extinction-Escape
- “Everything You Always Wanted to Know about Sex (but Were Afraid to Ask)” in after Two Decades of Laboratory and Field Analyses
- Norovirus Gastroenteritis, Carbohydrate Receptors, and Animal Models
- Variations in TcdB Activity and the Hypervirulence of Emerging Strains of
- SWAN-1 Binds to EGL-9 and Regulates HIF-1-Mediated Resistance to the Bacterial Pathogen PAO1
- Conformational Adaptation of Asian Macaque TRIMCyp Directs Lineage Specific Antiviral Activity
- The Proteasome Active Site Threonine Is Essential for Persistence Yet Dispensable for Replication and Resistance to Nitric Oxide
- Characterization of Oseltamivir-Resistant 2009 H1N1 Pandemic Influenza A Viruses
- The Pneumococcal Serine-Rich Repeat Protein Is an Intra-Species Bacterial Adhesin That Promotes Bacterial Aggregation and in Biofilms
- Regulatory T Cell Suppressive Potency Dictates the Balance between Bacterial Proliferation and Clearance during Persistent Infection
- Structural Alterations in a Component of Cytochrome Oxidase and Molecular Evolution of Pathogenic in Humans
- A Limited Number of Antibody Specificities Mediate Broad and Potent Serum Neutralization in Selected HIV-1 Infected Individuals
- Spliced Leader Trapping Reveals Widespread Alternative Splicing Patterns in the Highly Dynamic Transcriptome of
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



