-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Toll-Dorsal Pathway Is Required for Resistance to Viral Oral Infection in
Pathogenic microbes can enter their hosts through different routes. This can have a strong impact on which host defensive mechanisms are elicited and in disease outcome. We used the model organism Drosophila melanogaster to understand how resistance to viruses differs between infection by direct virus entry into the body cavity and infection through feeding on food with the virus. We show that the Toll pathway is required to resist oral infection with different RNA viruses. On the other hand this pathway does not influence the outcome of viral infection performed by injection. Together our results show that the Toll pathway has a route-specific general antiviral effect. Our work expands the role of this classical innate immunity pathway and contributes to a better understanding of viral oral infection resistance in insects. This is particularly relevant because insect vectors of emerging human viral diseases, like dengue, are infected through feeding on contaminated hosts.
Published in the journal: The Toll-Dorsal Pathway Is Required for Resistance to Viral Oral Infection in. PLoS Pathog 10(12): e32767. doi:10.1371/journal.ppat.1004507
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004507Summary
Pathogenic microbes can enter their hosts through different routes. This can have a strong impact on which host defensive mechanisms are elicited and in disease outcome. We used the model organism Drosophila melanogaster to understand how resistance to viruses differs between infection by direct virus entry into the body cavity and infection through feeding on food with the virus. We show that the Toll pathway is required to resist oral infection with different RNA viruses. On the other hand this pathway does not influence the outcome of viral infection performed by injection. Together our results show that the Toll pathway has a route-specific general antiviral effect. Our work expands the role of this classical innate immunity pathway and contributes to a better understanding of viral oral infection resistance in insects. This is particularly relevant because insect vectors of emerging human viral diseases, like dengue, are infected through feeding on contaminated hosts.
Introduction
Pathogens can infect their hosts through many different routes. In humans, for instance, microbes can directly enter the host through skin lesions or mediated by insect vectors. However, most of human infections start at mucosal surfaces of the respiratory, digestive or genital tracts. Pathogens specialize in different transmission strategies involving different host tissues. On the other hand, hosts mount distinct immune responses in different tissues, involving specialized cells and structures. Therefore, pathogen entry route can have a strong impact on the result of infection in animals, from humans to insects [1]–[4].
In Drosophila melanogaster oral or systemic infection with bacteria trigger different responses and have different outcomes (see [5] for review). Injection of bacteria into the haemocoel induces a systemic immune response based on the secretion of proteins into the haemolymph by the fat body [6]–[9]. Oral infections prompt a local immune response in the gut, and in some cases also a systemic response [10]–[13]. In both these responses the immune deficiency (Imd) signaling pathway can be activated and many antimicrobial peptides are produced [13]. However, these responses differ in other activated pathways and induced genes [13], [14]. Notably, the Toll pathway, a major mediator of systemic immune responses, is not involved in the gut local response. Injection of bacteria is generally more pathogenic than oral infection, with lower titres of bacteria being required for a lethal effect [2], [15]. Interestingly, the bacteria Serratia marcescens administered through oral infection can cross the gut barrier and enter the haemolymph, however these systemic bacteria have a lower pathogenicity than corresponding titres directly injected [15]. These findings indicate that natural infections lead to more structured and effective immune responses. These functional differences are also reflected in evolutionary processes since Drosophila adaptation to pathogenic bacteria is dependent on infection route [2].
Viral infections in insects have also been show to differ with infection route. For example, honeybees infected by Deformed Wing virus (DWV) through vertical transmission or horizontal oral transmission have no apparent disease symptoms. However, if horizontally transmitted by the parasitic Varroa mite, presumably from the mite saliva to the bee haemocoel, DWV is highly pathogenic [16]–[18]. Understanding the common and unique characteristics of insect defence against viral pathogens delivered through different routes is important in order to explain this differential pathogenicity. Moreover, resistance to viral oral infection in insects is also of particular interest since vectors of arboviruses are mainly infected through feeding on contaminated hosts.
Drosophila melanogaster has become an important model organism to study innate antiviral immunity in insects [19]–[21]. Some Drosophila viruses are vertically transmitted (e.g. Sigma virus) [22] and others can be infective by feeding, such as Nora virus [23], [24] and Drosophila C virus (DCV) [25]–[29]. ERK has recently been shown to be involved in resistance to RNA viruses by oral infection [29]. However, most of D. melanogaster antiviral immunity research has been done on systemic infection with viruses. The best characterized antiviral mechanism in Drosophila is the RNA interference (RNAi) pathway that has a strong influence on infection by a wide range of viruses, including RNA and DNA viruses [30]–[35]. Consistent with the important role of RNAi, several viruses express suppressors of this mechanism [30], [32], [36]. Other important mediators of antiviral protection are the intracellular bacteria Wolbachia [37], [38]. Presence of these endosymbionts increases resistance to several RNA viruses [37]–[40].
The role of classical Drosophila inducible immune pathways in antiviral defence seems less broad or well defined. The JAK/STAT pathway is required for resistance to DCV and Cricket Paralysis virus (CrPV) but not to other viruses [35], [41]. Similarly, mutants in the Imd pathway are less resistant to Sindbis virus and CrPV [42], [43] but not to DCV [44]. The role of the Toll pathway in antiviral immunity is less clear. This pathway is initiated by the binding of the cytokine Spätzle to Toll which triggers an intracellular signalling cascade involving the adaptor proteins dMyD88 and Tube and the kinase Pelle, and leads to activation of the NF-κB transcription factors Dorsal and Dorsal-related immunity factor (Dif) [45]–[47]. These transcription factors are normally sequestered in the cytoplasm and translocate to the nucleus upon Toll pathway activation. No phenotype was observed with DCV or Sindbis in Dif mutants or Dif and dorsal double mutants, respectively [43], [44]. However, Dif mutants are more susceptible to Drosophila X virus (DXV) [48]. On the other hand, the role of the whole pathway in resistance to DXV is not clear since loss-of-function mutants in Toll (Tl), spätzle (spz), tube (tub) and pelle (pll) show no phenotype [48]. Moreover, constitutive activation of the pathway, in a Toll gain-of-function mutant, also leads to higher susceptibility to DXV [48].
Data in other insects support an antiviral role for the Toll pathway. In honeybees dorsal-1A knockdown increases titres of DWV [49]. Also, in the mosquito Aedes aegypti the Toll pathway is induced upon ingestion of a dengue virus infected blood meal and inactivation of the pathway resulted in increased viral loads [50]. These studies raise the possibility that the Toll pathway is generally involved in the response to viruses in insects and prompt further analysis of its function in Drosophila antiviral immunity.
Here we investigate the role of the Toll pathway in immune response to several RNA viruses on Drosophila melanogaster comparing a natural infection route (i.e. by feeding) and systemic infection. We show that several Toll pathway components, including the extracellular cytokine Spätzle, the membrane receptor Toll, the kinase Pelle and the NF-kB-like transcription factor Dorsal, are required to resist natural viral infections in Drosophila but not systemic infection. These data provide evidence that the inducible Toll pathway has a route-specific general antiviral effect.
Results
Characterization of DCV oral infection in Drosophila
DCV is a non-enveloped virus with a single-stranded, positive-sense RNA genome that belongs to the Dicistroviridae family [51]. This virus is a natural pathogen of D. melanogaster that can be found in both wild and laboratory fly populations [22]. On most Drosophila studies using DCV the virus is injected directly into the body cavity, bypassing putative natural barriers and immune defences. In order to infect Drosophila flies with DCV through a natural route, we developed a protocol for oral DCV infection in adults. The protocol consisted in keeping adult flies with a mix of DCV and yeast for 24 hours in a vial. After this period, defined as 0 days post-infection (dpi), flies were transferred to vials containing standard Drosophila food and their survival scored daily. We found that DCV oral infection in adult DrosDel w1118 isogenic (hereafter called w1118 iso) [52] flies can cause a lethal infection in both females and males, killing up to 25% of flies in 20 days (Fig. 1A, 1B and Dataset S1). We observed that flies started to die 5 to 6 dpi, similarly to infection by injection or pricking. We fitted the survival data with a Cox proportional hazard mixed effect model and compared the relative risk of dying of infected flies with non-infected controls (mock). In order to compare the different doses with each other we performed a Tukey's test on the resulting Cox hazard ratios. Lethality is dose-dependent since we observed that higher DCV doses induce significantly different higher lethality rates (Fig. 1A, 1B, S1 and Dataset S1).
Fig. 1. DCV oral infection can cause lethal infections. 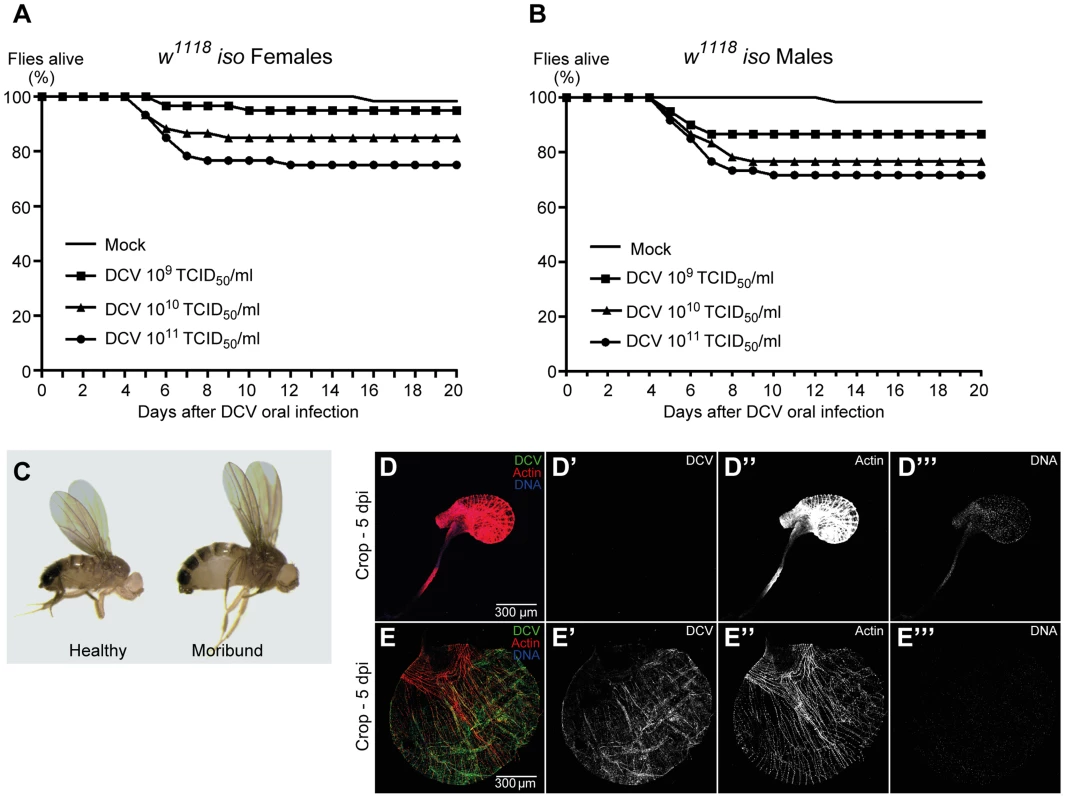
(A and B) Survival of adult flies after DCV oral infection. Sixty w1118 iso females (A) or males (B), 3–6 days-old flies were orally infected with DCV (109, 1010, or 1011 TCID50/ml) or buffer (Mock), and the survival was monitored daily. Three independent experiments were performed, with similar results. Survival data of both genders and the three experiments were analysed together using the Cox proportional hazard mixed effect model and observed that dose is a highly significant factor (p<0.0001). (C–E) Flies were infected with DCV at 1011 TCID50/ml. (C) After DCV oral infection moribund flies become inflated while challenged but healthy-looking flies do not. (D–E) Moribund flies (E) exhibit an oversized and overinflated crop when compared with challenged healthy-looking flies (D). In moribund flies crop muscle cells are infected with DCV (E). Adult male crops were immunostained with antibody against DCV (green), actin marked with phalloidin (red) and DNA marked with TOTO3 (blue). We observed that both females and males that become lethargic and inflated die within one day (Fig. 1C). In order to identify the reason of the observed overinflated body, particularly the abdomen, we dissected these flies at 5 dpi. Moribund flies exhibit an oversized crop when compared with healthy flies (Fig. 1D, 1E and S2). Using immunofluorescence we detected DCV infecting crop-associated muscle cells (Fig. 1E), suggesting that viral infection of this visceral muscle is the reason of the crop oversize.
To further characterize the course and the tropism of DCV upon oral infection we investigated which tissues were infected at 0 dpi (immediately after the 24 hours DCV exposure), 2 dpi and 5 dpi. We analysed oesophagus, crop, proventriculus, midgut, Malpighian tubules, hindgut, male and female reproductive organs, haemocytes, fat body, trachea and thorax skeletal muscle. At 0 dpi we were able to detect virus particles in the lumen of the midgut (Fig. 2A), indicating that the virus is reaching at least as far as the midgut. However, we were not able to detect any DCV infected cell, including epithelial and visceral muscle cells of the midgut (Fig. 2A). At 2 dpi the only tissue in which we could detect infection was the fat body (Fig. 2B). This DCV infection was confined to some regions of the fat body, mostly in the abdominal region. At 5 dpi DCV was also detected in the fat body (Fig. S3A) and the extent of the infection there was much greater than at 2 dpi. Analysis of flies 5 dpi also revealed the presence of DCV in the visceral muscle surrounding the crop, midgut and hindgut (Fig. 1E, 2C and 2D). Despite the presence of DCV in the midgut visceral muscle, we were not able to detect any virus in the gut epithelium. We also detected DCV in the muscle surrounding the Malpighian tubules at the junction point with the gut, but we were not able to detect DCV in the Malpighian tubules cells (Fig. S3B). The visceral muscle cells of the ovarian and testis peritoneal sheaths were also infected with DCV (Fig. 2E and F). We detected DCV in the abdominal muscle rarely (less than 1/40 flies) (Fig. S3C) but we never found DCV in thorax skeletal muscle (Fig. S3D). DCV was also detected in small sections of the tracheal system, mostly frequently in the abdominal region (Fig. S3E). Additionally, we observed that DCV was present in some circulating haemocytes (Fig. 2G). This could indicate that DCV efficiently infects haemocyte cells. Another possible explanation is that haemocytes phagocytose infected cells. Overall, these immunofluorescence results show that by oral infection, DCV infects specific tissues of D. melanogaster.
Fig. 2. DCV tissue tropism upon oral infection. 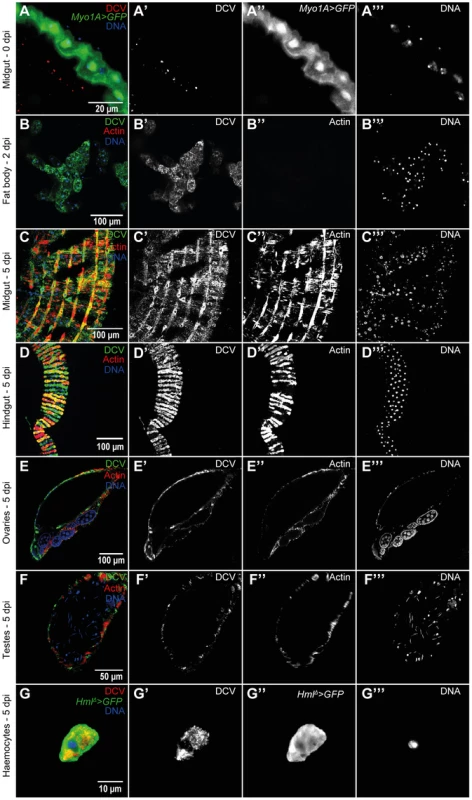
(A) DCV is present in midgut lumen at 0 dpi. Adult male guts were immunostained with antibody against DCV (red), epithelial enterocytes were marked with GFP expression (green) driven by Myo1A-Gal4 and DNA marked with TOTO3 (blue). (B) Fat body is infected with DCV 2 dpi. Midgut (C) and hindgut (D) muscle cells are infected with DCV at 5 dpi. Muscle cells of the ovarian (E) and testis (F) peritoneal sheath are also infected with DCV 5 dpi. (B–F) DCV was immunostained with an antibody (green), actin marked with phalloidin (red) and DNA marked with TOTO3 (blue). Haemocytes (G) are infected with DCV 5 dpi. Haemocytes were marked with GFP expression (green) driven by hml(delta)-Gal4, DCV was immunostained with an antibody (red), and DNA marked DAPI (blue). All tissues were dissected from adult flies. DCV infections (1011 TCID50/ml) were performed in 3–6 days old flies. In order to compare DCV tropism upon oral and systemic infections we examined all the above tissues in 20 males 2 and 5 dpi for both infection protocols using immunofluorescence. For this we pricked flies with a relatively low dose of DCV (105 TCID50/ml). Analysis of orally infected flies confirmed that at 2 dpi only the fat body was infected with DCV (Fig. 3A and Table 1). There was a restriction to the fat body in some systemically infected flies at 2 dpi, however in other flies the infection was also present in other tissues (Fig. 3B and Table 1). At 5 dpi DCV was detected in the same tissues for both virus-delivery methods (Fig. 3C, 3D and Table 1). These results show that independently of delivery route DCV tropism is largely the same, although it is less restricted to the fat body in the early stages of systemic infection.
Fig. 3. DCV oral and systemic infections have similar tissue tropism. 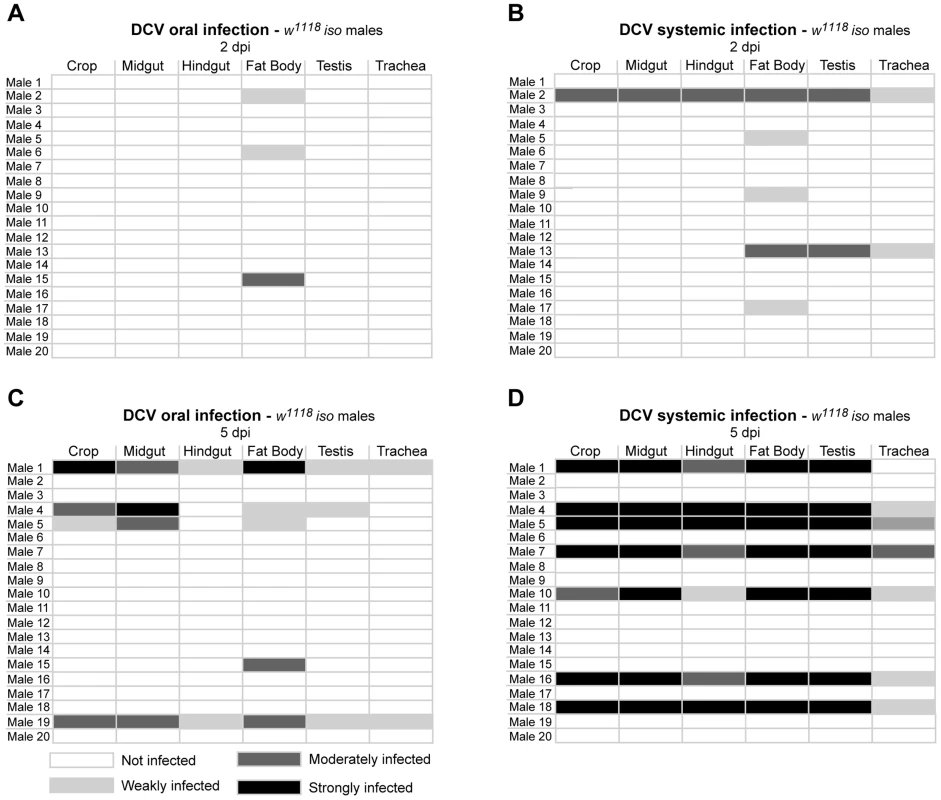
(A and B) DCV tissue tropism 2 days after oral (A) and systemic (B) infection. (C and D) DCV tissue tropism 5 days after oral (C) and systemic (D) infection. DCV was at 1011 TCID50/ml for oral infection, 105 TCID50/ml for systemic infection. Tissues of twenty adult males per condition were dissected and immunostained with an antibody against DCV, actin marked with phalloidin and DNA marked TOTO3. Oesophagus, crop, proventriculus, midgut, Malpighian tubules, hindgut, testes, fat body, trachea and thorax skeletal muscle tissues of every individual was analysed for DCV presence and the intensity of the infection by confocal microscopy. “Not infected” - DCV not detected in any part of the tissue observed. “Weakly infected” - DCV was detected in less than one third of the tissue. “Moderately infected” - DCV was detected in one to two thirds of the tissue. “Strongly infected” - DCV was detected in more than two thirds of the tissue. DCV infections were performed in 3–6 days old flies. Tab. 1. Presence of DCV in haemocytes of infected flies. 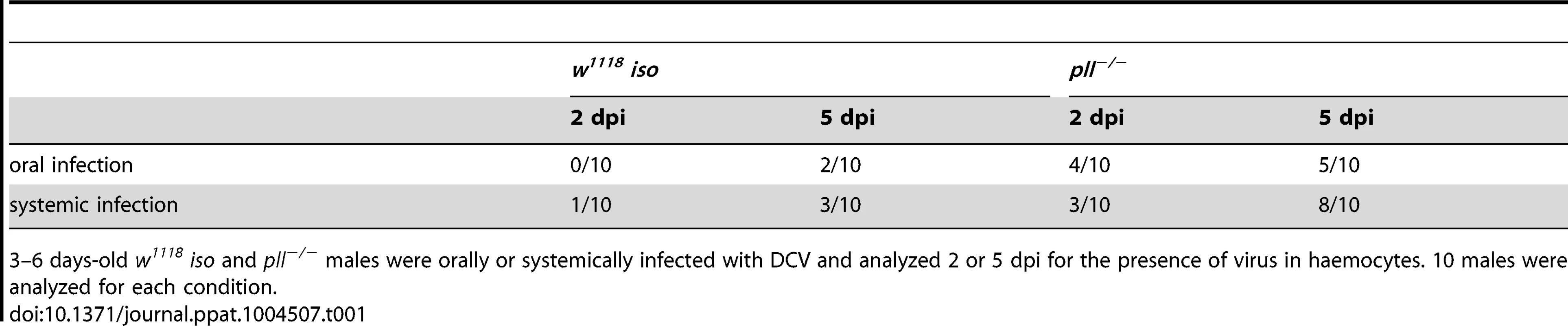
3–6 days-old w1118 iso and pll−/− males were orally or systemically infected with DCV and analyzed 2 or 5 dpi for the presence of virus in haemocytes. 10 males were analyzed for each condition. Toll pathway mutant flies are less resistant to DCV oral infection
In order to analyse the role of the Toll pathway in the response to viral oral infection we tested a collection of mutants in different genes of the Toll pathway: spz (spz4/spz4), Tl (Tlrv1/Tlr3), pll (pll2/pll21), dorsal (dl1/dl1) and Dif (Dif1/Dif1). To limit putative effects of different genetic backgrounds each mutation was introgressed into the w1118 iso background. This introgression was done by chromosome replacement and backcrossing (see Materials and Methods). We orally infected these mutant lines with DCV and their survival was compared to the control line w1118 iso. Flies mutant in the genes spz, Tl and pll were more susceptible to DCV oral infection than w1118 iso control flies (Fig. 4A, 4B, S4A, S4B and Datasets S2, S3, S4). For pll mutants we further show increased sensitivity, compared with w1118 iso, at several DCV infection doses and dose-dependent lethality (Fig. S5A–D and Dataset S5). Mutations in the genes encoding the two NF-κB homologues known to be downstream of the Toll pathway give different results. Dif mutants do not show a phenotype in this assay and are as sensitive to DCV infection as the w1118 iso control flies while dl mutants show high susceptibility, to the same degree as spz and pll mutants (Fig. 4A and S4A). These results contrast with the requirement of Dif but not dl in adult flies to resist bacteria and fungi [46], [53]–[56]. The high lethality observed for the several mutations in genes of the Toll pathway when compared to the control background are a consequence of DCV infection, since in the absence of viral infection and in the timeframe of this analysis these mutations have no effect on survival, except for the dl mutant that seems to be slightly deleterious by itself (Fig. 4C and S4C). In summary, these data show that the Toll pathway is important to survive DCV infection and Dorsal, but not Dif, is the downstream transcription factor required.
Fig. 4. Toll Pathway mutant flies are less resistant to DCV oral Infection. 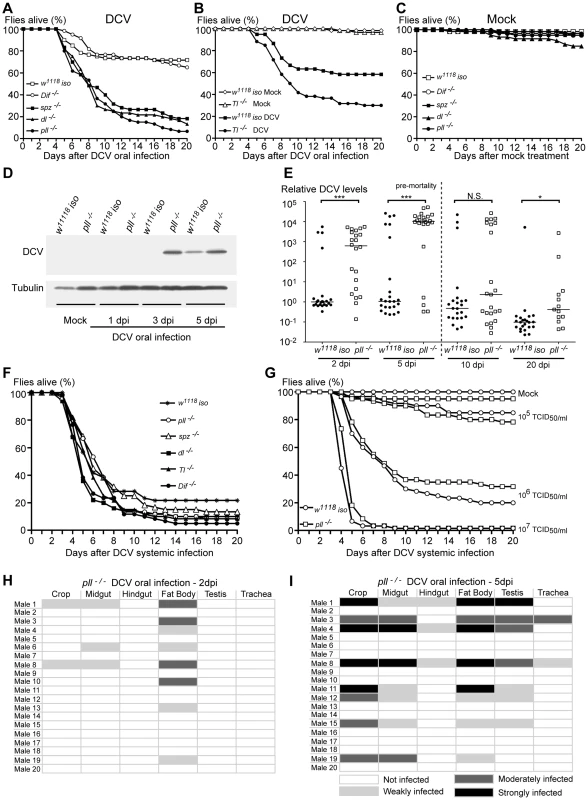
(A and B) Survival of Toll pathway mutants upon DCV oral infection (1011 TCID50/ml) or buffer. Male flies spz−/− (spz4/spz4), pll−/− (pll2/pll21), dl−/− (dl1/dl1), Dif−/− (Dif1/Dif1) (A) and Toll−/− (Tlrv1/Tlr3) (B) were compared to w1118 iso. spz−/−, pll−/− dl−/− and Tl−/− flies were significantly different from w1118 iso (Cox proportional hazard mixed effect model, p<0.001 for all four lines). Dif−/− mutant flies were not significantly different from w1118 iso (p = 0.331). (C) Survival of Toll pathway mutants upon mock treatment. None of the mutant lines were significantly different from w1118 iso (Cox proportional hazard mixed effect model, p>0.67), except dl (p = 0.003). (D) DCV protein levels after oral infection. 3–6 days old males of pll−/− and w1118 iso lines were orally infected with DCV (1011 TCID50/ml), collected 1, 3 and 5 days later for protein extraction, and probed in a Western blot with anti-DCV antibody (10 flies per sample). Anti-tubulin antibody was used as a loading control. (E) DCV RNA levels upon oral infection. 3–6 day old males of pll−/− and w1118 iso lines were orally infected with DCV (1011 TCID50/ml) and collected 2, 5, 10 and 20 days later for RNA extraction and RT-qPCR. 10 and 20 dpi infection samples are biased since they were collected after the major peak of DCV-induced mortality and therefore most highly infected flies have presumably died. Relative amount of DCV was calculated using host Rpl32 mRNA as a reference and values are relative to median of w1118 iso samples at 2 dpi. Each point represents a sample (one male), and lines are medians of the samples. DCV loads are significantly different between pll−/− and w1118 iso line at 2, 5 and 20 dpi (Wilcoxon test, p<0.001, p<0.005, p = 0.25 and p<0.05 for 2, 5, 10 and 20 dpi, respectively). (F) Survival of Toll pathway mutants upon DCV systemic infection (pricked at 107 TCID50/ml). None of the mutant lines were significantly different from w1118 iso (Cox proportional hazard mixed effect model, p>0.1). (G) Survival of pll−/− and w1118 iso male flies to different doses of DCV systemic infection. (105, 106 and 107 TCID50/ml). pll−/− flies were not significantly different from w1118 iso (Cox proportional hazard mixed effect model, p = 0.840, p = 0.626 and p = 0.085, respectively). (H and I) DCV tissue tropism of pll−/− flies upon oral infection, 2 dpi (H) and 5 dpi (I). Twenty adult males per condition were dissected and immunostained with an antibody against DCV and analysed as before. (A, B, C, F, G) For all survival experiments, sixty 3–6 days old males, per line or condition, were infected with DCV or buffer, and their survival was monitored daily. Survival assays for oral infections were performed thrice for pll, spz, an dl mutants, and twice for Dif and Tl mutants. Survival assays of systemic infection in panel F were performed twice. Survival data of all replicates were analysed together using Cox proportional hazard mixed effect models. To investigate whether the increased lethality rates of pll-deficient flies was due to decreased resistance or tolerance to DCV we analysed the viral levels by Western blot at 1, 3 and 5 dpi. We observed that at 3 and 5 dpi pll mutant flies had more viral protein than w1118 iso flies (Fig. 4D and S6). We confirmed these results by measuring DCV RNA levels of single flies at 2, 5, 10 and 20 dpi using reverse transcription quantitative PCR (RT-qPCR). A greater number of pll mutant flies exhibited high quantities of DCV RNA when compared with w1118 iso flies at 2 and 5 dpi (Fig. 4E, S5E and Dataset S6). DCV titres are significantly different between these lines at these days and the median of viral RNA load was approximately one thousand to ten thousand times higher in pll mutant flies (Fig. 4E and S5E). All the flies analysed are infected with DCV, even the ones that survive the infection for 20 days. This shows that there is no clearance of the virus in this timeframe. These results show that a mutant in the Toll pathway has lower resistance to DCV upon oral infection.
In order to investigate if the Toll pathway is also required to resist DCV systemic infection we pricked Toll pathway mutants and w1118 iso flies with DCV and followed their survival for 20 days. We found that Toll pathway mutant lines were not more susceptible to DCV systemic infection when compared with w1118 iso flies (Fig. 4F, S7A–C and Datasets S7, S8). We further analysed the pll mutant infected at several doses in order to rule out a dose-specific lack of effect. pll mutant was not more sensitive than w1118 iso to DCV systemic infection in any of the doses (Fig. 4G and Dataset S9). These results suggest that, contrary to oral infection, the Toll pathway is not important in the immune response to DCV systemic infection.
To explore whether Toll pathway mutant flies have altered patterns of infection, we analysed DCV tropism at 2 and 5 dpi in pll mutant flies after oral infection. As before, 20 males were analysed per time point. In this mutant we can detect DCV in a higher proportion of flies at 2 dpi and 5 dpi than w1118 iso (40% compared with 15 or 25%, Fig. 3A, 3C, 4H, 4I and Table 1), in agreement with the RT-qPCR data. In contrast to w1118 iso flies (Fig. 3A), at 2 dpi DCV is not restricted to the fat body and can also be detected in muscle surrounding the crop and the midgut (Fig. 4H). At 5 dpi pll mutant flies showed the same DCV tropism as the observed in w1118 iso (Fig. 3C and 4I). We were also unable to detect DCV in crop or midgut epithelial cells in pll mutants. These results show that although DCV seems to be spreading faster in pll mutant flies than in w1118 iso, overall there is no difference in DCV tropism.
Lack of interaction between Wolbachia and other microbiota with Toll resistance to viruses
Wolbachia induces resistance to infection by RNA viruses in D. melanogaster and other insects [37], [38], [57]. In mosquitoes this protection has been suggested to be dependent on the Toll pathway [58]. To test this hypothesis in D. melanogaster we compared the survival of w1118 iso and pll-deficient flies, infected and non-infected with Wolbachia. The results show that in D. melanogaster Wolbachia also protects against viral oral infection (Fig. 5A, S8A–C and Datasets S10, S11, Cox Proportional Hazards Model, p<0.001). We observed that Wolbachia protects pll mutants against DCV infection to the same extent as w1118 iso flies (Fig. 5A and S8A–C). The pll mutation does reduce survival of Wolbachia-positive flies when orally infected with DCV but to the same extent as in Wolbachia-free flies and there is no interaction between the two factors (Cox Proportional Hazards Model, Wolbachia*Genotype interaction; p = 0.67). The same lack of interaction is observed for systemic DCV infection (p = 0.69). Therefore in D. melanogaster the Toll pathway is not absolutely required for Wolbachia protection to DCV, confirming previous data with dengue virus [59].
Fig. 5. Lack of interaction between Wolbachia and other microbiota with Toll resistance to viruses. 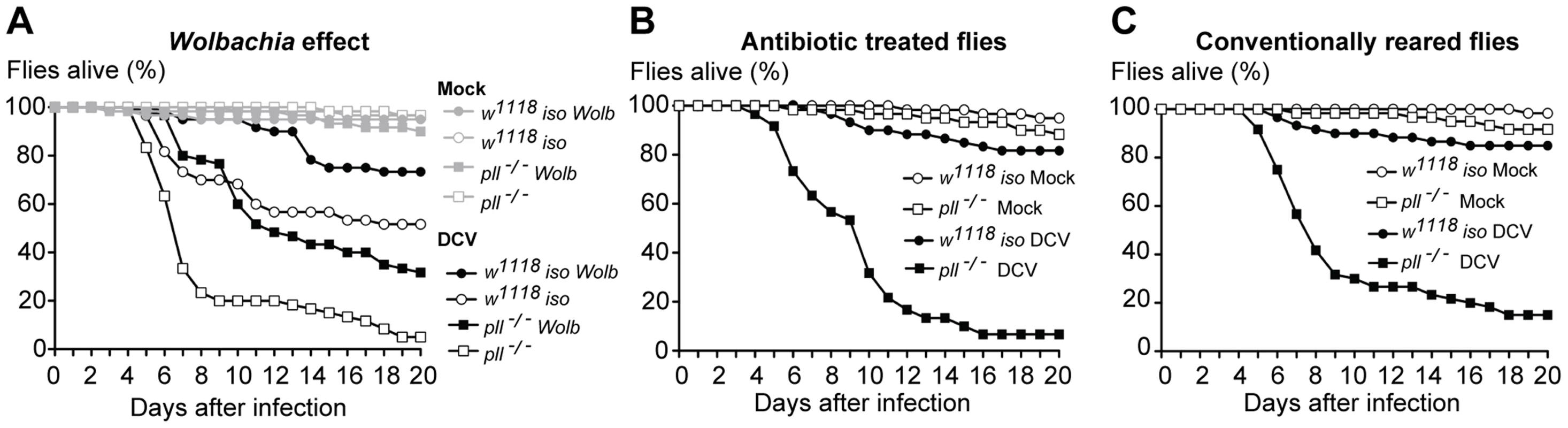
(A–C) Sixty 3–6 days old males of each line were orally infected with DCV (1011 TCID50/ml) or buffer (Mock), and survival was monitored daily. Survival data was fitted with a Cox proportional hazard mixed effect model. (A) Wolbachia protection to DCV oral infection does not require the Toll pathway. There is no interaction between Wolbachia and genotype (p = 0.67). (B–C) Survival of antibiotic treated (B) and conventionally reared (C) pll−/− and w1118 iso flies after DCV oral infection. There is no effect of antibiotic treatment in fly survival (p = 0.28). pll−/− flies show increased mortality relative to w1118 iso flies in both antibiotic treated or conventionally reared conditions (p<0.001 in both conditions). Other Drosophila-associated microbiota could also indirectly affect DCV oral infection and Toll pathway mediated protection. The Toll pathway could, for instance, be important to control a secondary infection with bacteria upon viral infection induced damaged. w1118 iso and pll-deficient flies were raised and maintained with antibiotics and susceptibility to DCV was compared with conventionally-reared flies (Fig. 5B and C, S8D–F and Dataset S12). There is no significant effect of the antibiotic treatment on the susceptibility to viruses (Cox Proportional Hazards Model; p = 0.28) and pll-deficient flies are still more susceptible to DCV infection in the absence of bacteria (Cox Proportional Hazards Model; p<0.001). Hence, in our experimental setup the Drosophila-associated microbiota does not play a role in the susceptibility to DCV oral infection or Toll-mediated resistance.
Toll pathway mutant flies are less resistant to oral infection with several viruses
To test the specificity of the Toll pathway in D. melanogaster antiviral immune response, we tested its requirement for resistance to other insect RNA viruses. Cricket Paralysis virus (CrPV) is closely related to DCV, also belongs to the Dicistroviridae family, and causes a lethal infection in adult flies [32], [42], [60]. Upon CrPV oral infection we observed that pll-deficient flies were more susceptible than control flies (Fig. 6A, S9A and Dataset S13). As with DCV oral infections, we found that a greater number of pll mutant flies exhibited higher amounts of CrPV RNA when compared with w1118 iso flies (Fig. 6B and Dataset S14). pll-deficient flies showed the same susceptibility to CrPV systemic infection as control flies at different viral infection titres (Fig. 6C, S9B and Dataset S15).
Fig. 6. Toll Pathway mutant flies are less resistant to other RNA viruses oral infection. 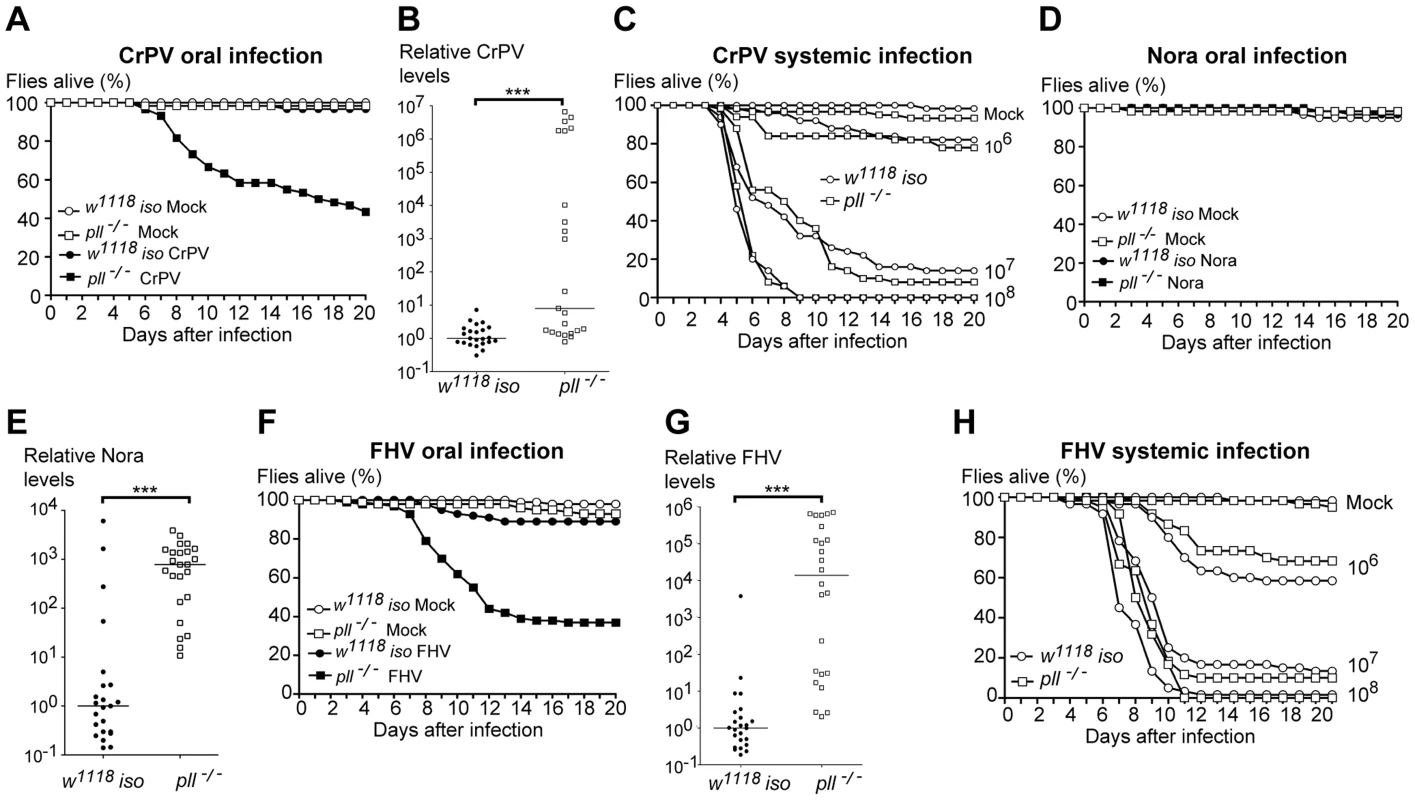
(A) Survival of pll−/− and w1118 iso flies after CrPV oral infection (1.76×1010 TCID50/ml) or buffer. pll−/− flies were significantly more sensitive to CrPV than w1118 iso (Cox proportional hazard mixed effect model, p<0.001). (B) CrPV RNA levels in pll−/− and w1118 iso flies upon oral infection (1.76×1010 TCID50/ml). CrPV loads are significantly different between pll−/− and w1118 iso line (Wilcoxon test, p<0.005). (C) pll−/− and w1118 iso flies were systemically infected with CrPV at three different concentrations (106, 107, 108 TCID50/ml). pll−/− mutant flies were not more susceptible to CrPV systemic infection than w1118 iso control flies (Cox Proportional Hazards Model, p = 0.966, p = 1.000 and p = 0.974, respectively). (D) Survival of pll−/− and w1118 iso flies upon Nora oral infection or buffer. pll−/− flies were not more sensitive than w1118 iso (Cox proportional hazard mixed effect model, p = 0.887). (E) Nora RNA levels upon oral infection. Nora loads are significantly different between pll−/− and w1118 iso line (Wilcoxon test, p<0.005). (F) Survival of pll−/− and w1118 iso flies upon FHV oral infection (1010 TCID50/ml) or buffer. pll−/− flies were significantly more sensitive than w1118 iso (Cox proportional hazard mixed effect model, p<0.001). (G) FHV RNA levels upon oral infection (1010 TCID50/ml). FHV loads are significantly different between pll−/− and w1118 iso line (Wilcoxon test, p<0.005) (in the other independent replicate the difference in medians is 20-fold and p = 0.05). (H) pll−/− and w1118 iso flies were systemically infected with FHV at three different concentrations (106, 107, 108 TCID50/ml). pll−/− mutant flies were not more susceptible to FHV systemic infection than w1118 iso control flies (Cox Proportional Hazards Model, p = 0.819, p = 0.709 and p = 0.225, respectively). For survival experiments (A, C, D, F and H) sixty 3–6 days old males of each line per treatment were used and survival was scored daily. Survival experiments for oral infections were performed thrice, yielding similar results. Survival data of all replicates was analysed together using the Cox proportional hazard mixed effect model. For viral loads experiments (B, E, G) 3–6 days old males of each line were orally infected with the virus of interest and collected 5–6 dpi for RNA extraction and RT-qPCR. Relative amount of virus was calculated using host Rpl32 mRNA as a reference and values are relative to the median of the w1118 iso samples. Each point represents the relative virus amount of a single fly and lines are medians of these values. All viral loads experiments were performed twice yielding similar results. We also tested whether pll-deficient flies are more sensitive to Nora virus oral infections. Nora virus is a picorna-like, non-enveloped virus, with a positive-sense single-stranded RNA genome [23]. This virus naturally infects D. melanogaster and causes persistent infection without any evidence of pathology [23], [24]. Nevertheless, we compared the lethality rates of pll-deficient flies with w1118 iso control flies upon Nora virus oral infection. As show in Fig. 6D and S9C (Dataset S16), we did not observe any lethality associated with Nora oral infection, even in pll mutants. However, when we measured Nora RNA levels of single flies 5 dpi we found that a greater number of pll mutant flies exhibited high amounts of viral RNA when compared with w1118 iso flies (Fig. 6E and Dataset S14).
Finally, we investigated the importance of Toll pathway in the immune response to Flock House virus (FHV). FHV is a non-enveloped, positive-sense RNA virus that belongs to the Nodaviridae family of insect virus [61]. Although FHV is not a natural pathogen of D. melanogaster it can replicate and cause lethality in adults when injected [31]. pll-deficient flies were more susceptible to FHV oral infection than w1118 iso control flies (Fig. 6F, S9D and Dataset S17) and had higher levels of viral RNA (Fig. 6G and Dataset S14). We also tested whether pll mutant flies were more susceptible to FHV systemic infection. In concordance with the DCV results, pll mutant flies were not more susceptible to FHV systemic infection when compared with w1118 iso flies across several doses of infection (Fig. 6H, S9E and Dataset S18).
These analyses with different viruses indicate that the Toll pathway is required to resist a broad range of RNA viruses. Moreover, this requirement seems to be specific to oral infection and not relevant in the context of a systemic infection. To establish whether the increased sensitivity to viral infection extended to other pathogens, we orally infected pll-deficient flies with Pseudomonas entomophila and compared their survival to w1118 iso. pll-mutant flies were not more susceptible to these Gram-negative bacteria than w1118 iso (Fig. S10A and Dataset S19). This was expected since the Toll pathway is not required for the transcriptional immune response to Gram-negative bacteria gut infection [13]. We also analysed the feeding rates of pll mutant and w1118 iso flies. When exposed to DCV mixed with yeast both lines had the same feeding rate (Fig. S10B and Dataset S20). These data show that Toll pathway mutant flies are not generally more susceptible to oral infection by all pathogens. Testing further pathogens will allow assessing if increased susceptibility of Toll pathway mutants is restricted to viruses.
DCV infection induces activation of the Toll pathway
Since we observed that flies mutant in genes of the Toll-Dorsal pathway have increased sensitivity to DCV oral infection, we investigated whether Dorsal is activated during viral infection. We probed if Dorsal was translocated from the cytoplasm to the nucleus upon DCV infection by using an antibody specific against its C-terminal domain [62] (Fig. S11A and B). At 5 days after oral infection, but not 2 dpi, we were able to detect nuclear import of Dorsal in fat body cells infected with DCV (20 flies were analysed in each time point) (Fig. 7A). This is only observed in infected cells although many fat body cells infected with DCV do not show Dorsal nuclear localization (Fig. 7B). However, we never detected Dorsal enrichment in the nuclei of non-infected fat body cells, even in infected flies (Fig. 7C and S11A). This nuclear translocation upon DCV infection seems specific to the fat body since we do not observe it other tissues, including gut epithelial and muscle cells (in the same 2 dpi and 5 dpi samples) (Fig. S11C and S11D). We have also failed to detect Dorsal translocation in haemocytes of w1118 iso flies orally or systemically infected (the w1118 iso infected flies analysed in Table 1) (Fig. S11E). We also analysed Dorsal localization after DCV systemic infection (2 dpi and 5 dpi, 10 flies each). Dorsal is translocated to the nuclei of fat body cells in response to DCV infection at 2 dpi (Fig. 7D) but not in epithelial or muscle cells of the gut in the same flies. Finally, we tested Dorsal translocation upon oral viral infection in a pll mutant line (Fig. 7E). We do not see Dorsal translocation in 16 DCV infected flies that are pll−/− but we see translocation in 4 out of 14 infected w1118 iso control flies (chi-square test, p = 0.037). This shows that Dorsal translocation in response to viral infection is dependent on the Toll pathway. In summary, these results show that Dorsal is translocated from the cytoplasm to the nucleus in fat body cells in response to DCV infection, suggesting that the Toll pathway is involved in an antiviral inducible immune response.
Fig. 7. Toll pathway activation by DCV infection. 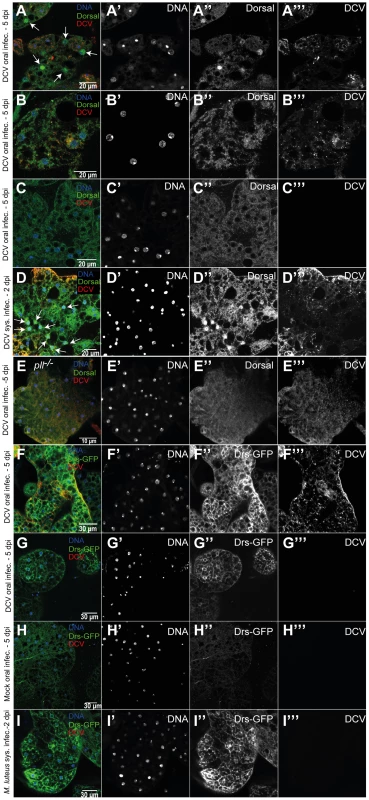
(A–C) Dorsal localization in fat body cells 5 days after DCV oral infection (1011 TCID50/ml) of w1118 iso flies. All three fat body regions shown were dissected from the same fly and are representative of 14 DCV-positive flies (out of 20 total flies analysed). (A) DCV infected fat body region with nuclear import of Dorsal (white arrows). Nuclear import of Dorsal was observed in 4 out of the 14 DCV positive flies (B) DCV infected fat body region without nuclear import of Dorsal. (C) Fat body region not DCV infected without nuclear import of Dorsal. (D) Dorsal localization in fat body cells 2 days after DCV systemic infection (107 TCID50/ml) of w1118 iso flies, showing nuclear import of Dorsal (white arrows). Nuclear import of Dorsal was observed in 5 of 10 DCV positive flies. (E) Dorsal localization in fat body cells of pll−/− flies after DCV oral infection (Dorsal nuclear import was seen in 0 out of 16 DCV positive flies) (A–E) Adult male fat body was immunostained with an antibody against Dorsal (green), an antibody against DCV (red), and DNA was marked with DAPI (blue). (F–G) Drs-GFP expression in fat body 5 days after DCV oral infection. Both fat body regions shown were dissected from the same fly. (H) Drs-GFP expression in fat body after 5 days mock oral infection. (I) Drs-GFP in fat body after 2 days Micrococcus luteus oral infection. (F–I) Adult male fat body regions were immunostained with antibody against DCV (red), antibody against GFP (green), and DNA marked with TOTO3 (blue). DCV infections were performed in 3–6 days old flies. Drosomycin (Drs) encodes an antimicrobial peptide and is a target gene of immune activation of the Toll pathway [7]. We probed expression of a Drs reporter gene [47] in response to viral infection. We observed Drs-GFP expression in the fat body of 8 out of 8 DCV infected flies but not in gut muscle or epithelium (Fig. 7F and G and S11F and G). Out of 8 non-infected flies none showed activation of Drs-GFP expression (Fig. 7H). The Drs-GFP fat body expression is present in infected and non-infected cells in DCV-infected flies, unlike Dorsal translocation, indicating a systemic activation of Toll pathway. This result further shows that the Toll pathway is activated in the fat body upon viral infection.
In order to test if inactivation of the Toll pathway in the fat body or other tissues (muscle, visceral muscle, enterocytes and haemocytes) would increase sensitivity to viruses we expressed three RNAi constructs for pll with different drivers and compared survival after DCV oral infection with control (Fig. S12 and Dataset S21). We failed to see any significant increase in lethality upon viral infection in these lines. Based on this negative result with RNAi it is not possible to conclude on the need of the Toll pathway anti-viral response in specific tissues.
Discussion
In Drosophila the Toll pathway plays a fundamental role in the response to systemic infection by fungi and bacteria [7], [54], [63]. Here we show that this pathway is also required to resist oral viral infections. Mutants in genes that encode the ligand Spätzle, the Toll transmembrane protein receptor, the cytoplasmic kinase Pelle and the NF-κB transcription factor Dorsal succumb faster to DCV infection and have higher titres of this virus. This demonstrates that a functional canonical Toll pathway is required for flies to survive a natural viral infection.
Two very similar NF-κB homologues, Dif and Dorsal, can be downstream of the Toll pathway in flies. Our genetic analysis shows that Dorsal but not Dif is required for viral resistance. In contrast, Dif but not Dorsal is required for adult systemic response to infection by bacteria and fungi [7], [46], [53], [63] and has been regarded as the Toll pathway transcription factor involved in adult immune systemic response. Nonetheless, other data also indicate a role for Dorsal in the immune response. In larvae both Dif and Dorsal are translocated into fat body cell nuclei upon systemic infection with bacteria [6], [45], [64] and Dorsal is upregulated in infected larvae [65]. In larvae Dif and Dorsal may be redundant in resistance to bacteria with the double mutant being very susceptible to normal Drosophila-associated microbiota [66]. Dif and Dorsal can form homo and heterodimers and these recognize different DNA sequences and differentially activate target genes [67], [68]. However, overexpression of one or the other transcription factor many times is sufficient to rescue mutant or double mutant phenotypes [66], [69] as well as activate expression of antimicrobial peptides [69]. Overall, the exact role and contribution of either transcription factor in the several types of immune responses of Drosophila is not known. On the other hand Dorsal also has a clear role in development in early embryogenesis, which Dif does not seem to share [45], [70]. The Toll pathway, although not necessarily through Dorsal, also has a role in muscular and neuromuscular development [71]–[75] and hematopoiesis [76]. This raises the possibility that the phenotypes we observed are due to development problems. This hypothesis would be particularly relevant for oral infection with viruses if development problems were to lead to alterations in feeding. However, we observe no differences in feeding rates between pll mutants and control. Moreover, we show that pll mutants are not more susceptible to oral infection with a bacterial pathogen. These data indicate that it is not a major digestive system development problem that leads to lower resistance to oral viral infection. We cannot absolutely rule out a development problem; however, we detect Dorsal translocation into the nuclei of DCV infected fat body cells and expression of a Drosomycin reporter gene in the fat body of infected flies. This shows that the Toll-Dorsal pathway is induced upon viral infection and, together with the genetic data, strongly supports a Toll pathway mediated anti-viral response. Identification of the target genes of the Dorsal transcription factor after viral infection will be important in the future, as well as understanding how they contribute to resistance to viruses.
During embryonic development and systemic immune response to fungi and bacteria the extracellular pro-Spätzle is proteolytically cleaved, leading to binding to the Toll receptor and activation of the pathway. In the case of infection, specific pattern recognition receptors present in the haemolymph are activated by microbial ligands and start a proteolytic cascade that culminates in pro-Spätzle cleavage [55], [56], [77]. Fungal and bacterial proteases can also lead to Spätzle cleavage through a different proteolytic cascade involving Persephone [77], [78]. At this point it is unclear how activation of the Toll pathway by viral infection works and it probably differs significantly from activation by bacteria and fungi. Putative pathogen associated molecular patterns associated with viruses and recognized by Drosophila must be different from the cell wall components of bacteria and fungi involved in Toll pathway activation. Moreover, viruses are intracellular parasites while the previously studied microbial elicitors of the Toll pathway are extracellular and present in the haemolymph. Previous work has shown that in Drosophila the Toll pathway also responds to tumours [79] and to a block in apoptosis, via Persephone [80], while in mosquitoes it can be activated by reactive oxygen species [58]. Viral infection could be indirectly detected by the Toll pathway through recognition of tissue damage and share a mechanism of activation with the above situations. Drs expression in response to viral infection is widespread in the fat body of infected flies and not restricted to infected cells. This indicates that Spätzle activation is systemic upon viral infection and that the Toll pathway is generally activated in the fat body of these flies. This is in agreement with previous published data showing up-regulation of Drs and Toll pathway genes upon DCV infection [28], [35]. As a further layer of complexity, our results show that Dorsal translocation is restricted to viral infected cells and is not observed throughout the fat body. This is at odds with a systemic activation of Spätzle and how the Toll pathway responds to bacteria and fungi. It is possible that Dorsal is activated throughout the fat body but that is not visible in the translocation assay. However, Dorsal activation and translocation to the nucleus may depend on Toll activation and a second cell-autonomous signal. In mammals RIG-I-like receptors (RLRs) and NOD-like receptors are involved in cell-autonomous activation of innate immunity in response to viral infection. There are no homologues of these cytoplasmic pattern recognition receptors in Drosophila. However, Dcr2 has a helicase domain homologous to helicase domains in RLRs and has been suggested to act as a pattern recognition receptor in Drosophila [81]. Toll-like receptors in mammals are also able to detect viral infection through binding to nucleic acids in vesicular compartments. Toll-7 in Drosophila can bind vesicular stomatitis viruses and induce antiviral autophagy [82]. Unravelling the signal that leads to Dorsal translocation in virally infected cells will be important to understand antiviral immunity in Drosophila.
Our results show that the increased lethality rates observed in the Toll pathway deficient flies are associated with higher DCV loads. Thus, the Toll pathway is involved in resistance to viruses. Furthermore, we demonstrate in this study that Toll requirement to control viral loads is not specific to DCV and extends to other RNA viruses, such as FHV, CrPV and Nora virus. Previous work did not see an effect of a pll mutant in a Nora virus infection assay [83]. The difference in our results may be due to different control of the genetic background or differences in the assay. We analysed the response to a new Nora virus oral infection while Habayeb and colleagues analysed the capacity to clear the viruses in a chronically infected Drosophila stock [83]. The median increase in viral titres we observe in pll mutants can be up to ten thousand fold. The magnitude of the difference is comparable or higher to differences between wild type flies and RNAi mutants [30]–[33] and between flies with and without Wolbachia [37]. The strength and generality of the interaction between the Toll pathway and viruses indicates that this is a major antiviral pathway in Drosophila. This is consistent with previous studies showing Toll pathway antiviral effect in mosquitoes and honeybees [49], [50].
The increased sensitivity to viruses in Toll pathway mutants is only manifested upon oral infection and not systemic infection. This is not a result of different infection titres with the two modes of infection because Toll pathway mutants are not more sensitive to a low dose of virus by systemic infection. Therefore, we have identified a pathway with a route-specific role. Nonetheless, we observe Dorsal nuclear translocation in fat body cells after both routes of infection. This indicates that the pathway is activated regardless of type of infection but it is only effective in a scenario of oral infection. In order to understand the differential requirement of the Toll pathway we performed a detailed analysis of the dynamics of DCV oral and systemic infections. Overall we found no major differences in the tissue distribution of DCV between the two infection routes. In both DCV is present in the fat body, trachea and visceral muscle of the crop, midgut and hindgut, and gonads. Although we can detect DCV particles in the midgut lumen shortly after oral ingestion, we could not determine its point of entry. We were unable to detect DCV infection in the epithelium of the digestive system at any time point. This could indicate that the DCV is transported across gut epithelial cells to the body cavity (haemocoel) without infecting the epithelial cells themselves. Transcytosis of virions has been described in mammals and insects [84]–[86]. An alternative explanation would be that DCV rapidly kills infected epithelial cells, therefore hindering their detection. Apoptosis of midgut cells following viral infection has been observed in Drosophila and in mosquitoes [87], [88]. However, a recent study in Drosophila reported that upon oral ingestion DCV was able to infect midgut epithelial cells [29]. The difference between these results may reflect differences in the feeding protocol: Xu and colleagues continually exposed flies to DCV for several days [29], while we only infect flies for one day. In our setup the fat body seems to be the first tissue to be infected; all infected flies have DCV in the fat body and some infected flies only have DCV in the fat body. This is more evident in orally infected flies that at 2 dpi only have DCV in this tissue. This may reflect a difference in the dynamics of the two infection routes and in systemic infected flies DCV seems to disseminate faster. The detection of Dorsal translocation only in fat body cells and the probable early restriction of DCV to this tissue when delivered by oral infection may be part of the explanation of the differential requirement of the Toll pathway in the two routes of infection.
Our results show that the Toll pathway is required to resist viral infections, which adds to the previously known requirement of the Toll pathway to resist bacteria, fungi, and parasitoids. This contributes to the idea that Spätzle may work more as a cytokine involved in general response to infection than to specific pathogens [5]. This Toll antiviral resistance is dependent on Dorsal and not Dif and we show Dorsal activation in virus-infected cells. The specificity of the immune response to difference pathogens may therefore rely on which transcription factors are activated downstream of the Toll pathway. Finally, we show that Toll requirement is restricted to viral oral infection and therefore route specific. This demonstrates that the interaction of viruses with Drosophila varies with mode of infection. Oral infection with viruses may be subject to more layers of control since it is probably the most frequent route of infection. Understanding this complexity is particularly relevant because arboviruses are transmitted to arthropod vectors of human diseases through feeding.
Materials and Methods
Fly strains and husbandry
Flies were maintained on standard cornmeal diet at a constant temperature of 25°C unless otherwise stated. All fly lines were cleaned of possible chronic viral infections as described elsewhere [22], [37]. Briefly, flies were aged to 30 days at 25°C and their eggs were collected in agar plates, treated with 50% bleach for 10 min, washed with water, and transferred to fresh vials.
Fly lines used in this study were free of Wolbachia except if otherwise stated. To mark midgut epithelial we used flies carrying the driver Myo1A-Gal4 (expressed in the enterocytes [89]) combined with UAS-GFP. We have analysed the following homozygous or heterozygous combination of mutants in the Toll pathway: spz4/spz4 (spz4 is a loss of function allele) [90], Tlrv1/Tlr3 (Tlrv1 is a loss of function allele and Tlr3 is a hypomorphic allele) [91], pll2/pll21 (pll2 is loss of function allele and pll21 is a hypomorphic) [90], [92], dl1/dl1(dl1 is a loss of function allele) [93], Dif1/Dif1 (Dif1 is a loss of function allele) [53]. To reduce genetic background effects these mutations were isogenized to the DrosDel w1118 isogenic background [52]. For each line the non-mutated chromosomes were replaced using balancer chromosomes whereas the mutation was recombined to the respective DrosDel w1118 isogenic chromosome for seven generations. We confirmed that the isogenized lines retained the mutation of interest by the associated development phenotype (lethality or maternal effect) or by DNA sequencing in the cases of absence of phenotype. For Drosomycin expression we used y w drs-GFP dpt-LacZ flies. For tissue specific pll knockdown the following drivers were used: C7-Gal4 (fat body driver [94]), 24B-Gal4 (visceral muscle driver [95]), Myo1A-Gal4 (midgut epithelium [89], [96]), mef2-Gal4 (somatic, visceral and cardiac muscle [97]) and hml(delta)-Gal4 (haemocyte driver [98]). Tlr3 (#3238) and dl1 (#3236) were obtained from the Bloomington stock center (http://flystocks.bio.indiana.edu/). Three independent UAS-pll-IR constructs and control UAS-mCherry-IR flies from TRiP collection [99] were used y1 sc* v1; P{TRiP.HMS01213}attP2 (#34733), y1 sc* v1; P{TRiP.GL00150}attP2 (#35577), y1 sc* v1; P{TRiP.HMS02332}attP40 (#41935), y1 sc* v1; P{VALIUM20-mCherry}attP2 (#35785). MyoIA-Gal4 was kindly given by Nicolas Tapon, spz4 and y w drs-GFP dpt-LacZ by Bruno Lemaitre, Tlrv1 by Kathryn Anderson, pll2 and pll21 by Steven Wasserman and Dif1 by Dominique Ferrandon.
Virus production and titration
DCV was produced either in cell culture or in flies. Cell culture DCV production and titration were performed as described in [37]. DCV production in flies was done in w1118 iso flies that were clear from viruses and Wolbachia infection [37], [100]. Flies were afterwards orally infected with DCV, which led to the establishment of a chronically infected stock. This stock was kept for at least five generations before extracting DCV from it. Because DCV infected stocks show a high lethality rate at pupal stage, we perform DCV extraction from pupae. We squashed 50 g of pupae in 50 ml of 50 mM Tris-HCl, pH 7.5. The extract was frozen at −80°C, thawed and centrifuged twice for 20 min at 27000 g at 4°C, keeping the supernatant. The supernatant was aliquoted and stored at −80°C and later titrated in Schneider's Line 2 (SL-2) cells as described in [37]. FHV and CrPV was produced and titrated in Schneider Drosophila line 2 (DL2) as in [37] and in [101], respectively, with minor changes. Nora virus extract was prepared from a naturally infected Oregon R stock [37]. One hundred adult flies were squashed in 1 ml of 50 mM Tris-HCl, pH 7.5. Extract was then frozen at −80°C, thawed and twice centrifuged for 10 min at 20000 g, at 4°C. The supernatant was aliquoted and stored at −80°C.
Viral infections and survival assays
Infections were performed on 3–6 days-old flies. To perform oral infection with virus we used empty plastic vials with 1×3 cm pieces of filter paper (Whatman gel blotting papper GB003) placed in the bottom. We loaded on the filter paper 350 µl of a mix of 75% virus extract and 25% of yeast (Saccharomyces cerevisiae, Sigma-Aldrich). Ten flies were placed per vial and left feeding for 24 hours at 25°C. For mock oral infections flies were exposed to buffer (50 mM Tris-HCl) mixed with yeast (25%). After this infection period we transferred the flies to new vials containing standard cornmeal diet. For viral systemic infections CO2 anesthetized flies were pricked in the thorax. The 0.15 mm diameter needles used for infection (Austerlitz Insect Pins) were dipped into a virus solution diluted to the desired concentration in 50 mM Tris-HCl, pH 7.5. After systemic infections flies were transferred to vials containing standard cornmeal food, 10 flies per vial. After both protocols of infection flies were kept at 25°C, checked for survival daily and vials changed every 5 days.
Bacteria infection
Pseudomonas entomophila was grown in LB at 30°C overnight. P. entomophila cultures were then concentrated by centrifugation and adjusted to OD600 = 75. For oral infections with P. entomophila flies were exposed to a 1∶1 solution of bacteria culture and 5% sucrose in water. In control mock infections, flies were exposed to LB with 5% sucrose. Survival was followed every 12 hours for 3 days. Micrococcus luteus was grown in LB at 37°C overnight, concentrated by centrifugation and adjusted to OD600 = 3. For systemic infections with M. luteus flies were pricked in the thorax with fine needles dipped in bacterial suspension. The P. entomophila and M. luteus strains used in this study were kindly provided by Bruno Lemaitre and Thomas Rival, respectively.
Immunostaining and microscopy
Flies were dissected to expose the internal tissues, fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min, washed in PBS, then incubated with 1% Triton-X-100 and 5% FBS in PBS (PTX-FBS) for 30 min. Samples were then incubated overnight with primary antibody at 4°C. Rabbit polyclonal antibodies raised against purified DCV (kindly given by Peter Christian) was used at 1∶200 dilution. Dorsal antibody developed by Ruth Steward was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242 [62], was used at 1∶5 dilution. The samples were washed with PTX-FBS, and then incubated in PTX-FBS with secondary antibodies conjugated with Alexa Fluor 488 or Alexa Fluor 568 (both by Molecular Probes) for 1 h. Samples were then washed with PTX-FBS, and incubated with Alexa Fluor 594 Phalloidin and DAPI or TOTO-3 (all by Molecular Probes) for 15 min. The samples were then washed in PTX-FBS, dissected and mounted in Vectashield Mounting Medium for microscopy. Confocal images were taken with either a Leica SP5 or Zeiss LSM 510 META confocal microscopes and processed in Fiji [102].
Western blots
3–6 day old males of each line were orally infected with DCV (1011 TCID50/ml), collected 1, 3 and 5 days later for protein extraction, and probed in a Western blot with anti-DCV antibody. Ten males were pooled per sample. Rabbit polyclonal antibodies raised against purified DCV was kindly given by Dr. Peter Christian. E7 mouse monoclonal anti-β-tubulin was acquired from Developmental Studies Hybridoma Bank [103].
RNA extractions and cDNA synthesis
For each sample RNA was extracted from one male fly using the Zymo Research Direct-zol RNA MiniPrep kit according to manufacturer's instructions. RNA concentrations were determined using NanoDrop ND-1000 Spectrophotometer. cDNA was prepared from 1 µg of total RNA using Random Primers and M-MLV Reverse Transcriptase (both Promega). Primers were allowed to bind to the template RNA for 5 min at 70°C and the reaction proceeded at 25°C for 10 min, 37°C for 60 min and 80°C for 10 min.
Real-time quantitative PCR
Each cDNA sample was analyzed in triplicate using a 7900HT Fast Real-Time PCR System (Applied Biosystems) instrument. We performed each reaction in a 384-well plate (Applied Biosystems), using 7 µl of iQ SYBR Green supermix (Bio Rad), 0,5 µl of each primer solution at 3,6 µM and 5 µl of diluted cDNA. Viral amplification was performed using the following thermal cycling protocol: initial 50°C for 2 min, denaturation for 10 min at 95°C followed by 40 cycles of 30 s at 95°C, 1 min at 56°C and 30 s s at 72°C. Melting curves were analysed to confirm specificity of amplified products. We obtained Ct values for manual threshold of 10 using the program SDS 2.4. Relative amounts of viral RNA were calculated by the Pfaffl Method [104] using Drosophila Rpl32 as a reference gene. The following primers were used: DCV forward 5′ - TCATCGGTATGCACATTGCT-3′; DCV reverse 5′-CGCATAACCATGCTCTTCTG-3′; FHV forward 5′ - ACCTCGATGGCAGGGTTT-3′; FHV reverse 5′ - CTTGAACCATGGCCTTTTG-3′; CrPV forward 5′-ACGAGGAAGCAACTCAAGGA-3′; CrPV reverse 5′-GAGCCCGCTGAGATGTAAAG-3′; Nora forward 5′-TTTCACTTTACTGTTGGTCTCC-3′; Nora reverse 5′-ATTCCATTTGTGACTGATTTTATTTC-3′; Rpl32 forward 5′ - CCGCTTCAAGGGACAGTATC-3′; Rpl32 reverse 5′-CAATCTCCTTGCGCTTCTTG-3′.
Germ-free like conditions
Flies w1118 iso and pll−/− were raised for one generation in food with a mix of antibiotics (100 µg/ml of streptomycin, 200 µg/ml of rifampicin and 100 µg/ml of tetracycline) [79], [105] and progeny was used to test susceptibility to virus. Flies were maintained in antibiotic food until the end of survival analysis. Elimination of bacteria was confirmed by plating homogenates of pll−/− flies that died during the time-course of infection. For each condition, a pool of 3 dead flies was homogenized with a pestle in 100 µl of LB. The homogenized extract was plated with the help of a 10 µl inoculation loop in Lactobacilli MRS broth and Mannitol culture media, which are able to grow Lactobacillus and Acetobacter, respectively [106]. The plates were incubated for 4 days at 25°C and subsequently scanned.
Statistics
All statistical analyses were done using R (2.10.1) [107].
To compare survival rates we used a Cox's proportional hazards mixed effect model (coxme in R). Fixed effects include sex, viral dose, genotype, presence/absence of Wolbachia, antibiotic treatment, and repeat of the experiment. To account for variation between vials of the same line in the same experiment, replicate vials were considered as a random effect. This method accounts for variation between vials of the same line in the same experiment and variation between replicates of the experiment.
To assess the significance of the different fixed factors and their interactions, we performed stepwise backward model selection, and compared the difference in the log-likelihood of the different models with a χ2 distribution, with the appropriate degrees of freedom.
To compare the different doses or the different genotypes with each other we performed either pairwise comparisons between all levels of the factors (Tukey-like contrasts [108]) or contrasted the genotype of interest with the respective genetic backgrounds, averaging for the effect of the remaining factors. When the interaction between factors was statistically significant, the factor of interest was compared independently for the different levels of the interacting variable. When needed, and in order to obtain independent estimates of the hazard ratios (e.g. between different genotypes, with and without infection), we calculated the hazard ratios in models which included the interaction term, despite the interaction being non-significant. Multiple comparisons were performed using the “multcomp” (function glht) and “lsmeans” (function lsmeans) packages in R.
In order to compare viral loads between genotypes we used a Wilcoxon rank sum test (wilcox.test in R).
To analyze the feeding rates we used a generalized linear model (GLM) with a binomial response, with the proportion of fed versus unfed flies as a dependent variable and genotype and time as fixed factors.
The p-value of the chi-square test (chisq.test in R) was computed for a Monte Carlo test with 109 replicates.
Supporting Information
Zdroje
1. LeggettHC, CornwallisCK, WestSA (2012) Mechanisms of pathogenesis, infective dose and virulence in human parasites. PLoS Pathog 8: e1002512 doi:10.1371/journal.ppat.1002512
2. MartinsNE, FariaVG, TeixeiraL, MagalhãesS, SucenaÉ (2013) Host adaptation is contingent upon the infection route taken by pathogens. PLoS Pathog 9: e1003601 doi:10.1371/journal.ppat.1003601
3. ClaytonDH, TompkinsDM (1994) Ectoparasite virulence is linked to mode of transmission. Proc Biol Sci 256 : 211–217 doi:10.1098/rspb.1994.0072
4. AgnewP, KoellaJC (1997) Virulence, parasite mode of transmission, and host fluctuating asymmetry. Proc Biol Sci 264 : 9–15 doi:10.1098/rspb.1997.0002
5. TeixeiraL (2012) Whole-genome expression profile analysis of Drosophila melanogaster immune responses. Brief Funct Genomics 11 : 375–386 doi:10.1093/bfgp/els043
6. LemaitreB, MeisterM, GovindS, GeorgelP, StewardR, et al. (1995) Functional analysis and regulation of nuclear import of dorsal during the immune response in Drosophila. EMBO J 14 : 536–545.
7. LemaitreB, NicolasE, MichautL, ReichhartJM, HoffmannJA (1996) The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86 : 973–983 doi:10.1016/S0092-8674(00)80172-5
8. KylstenP, SamakovlisC, HultmarkD (1990) The cecropin locus in Drosophila; a compact gene cluster involved in the response to infection. EMBO J 9 : 217–224.
9. WickerC, ReichhartJM, HoffmannD, HultmarkD, SamakovlisC, et al. (1990) Insect immunity. Characterization of a Drosophila cDNA encoding a novel member of the diptericin family of immune peptides. J Biol Chem 265 : 22493–22498.
10. TzouP, OhresserS, FerrandonD, CapovillaM, ReichhartJ-M, et al. (2000) Tissue-Specific Inducible Expression of Antimicrobial Peptide Genes in Drosophila Surface Epithelia. Immunity 13 : 737–748 doi:10.1016/S1074-7613(00)00072-8
11. Zaidman-RémyA, HervéM, PoidevinM, Pili-FlouryS, KimM, et al. (2006) The Drosophila amidase PGRP-LB modulates the immune response to bacterial infection. Immunity 24 : 463–473 doi:10.1016/j.immuni.2006.02.012
12. Bosco-DrayonV, PoidevinM, BonecaIG, Narbonne-ReveauK, RoyetJ, et al. (2012) Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune responses to infectious bacteria and tolerance to microbiota. Cell Host Microbe 12 : 153–165 doi:10.1016/j.chom.2012.06.002
13. BuchonN, BroderickNA, PoidevinM, PradervandS, LemaitreB (2009) Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5 : 200–211 doi:10.1016/j.chom.2009.01.003
14. VodovarN, VinalsM, LiehlP, BassetA, DegrouardJ, et al. (2005) Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc Natl Acad Sci U S A 102 : 11414–11419 doi:10.1073/pnas.0502240102
15. NehmeNT, LiégeoisS, KeleB, GiammarinaroP, PradelE, et al. (2007) A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog 3: e173 doi:10.1371/journal.ppat.0030173
16. YueC, GenerschE (2005) RT-PCR analysis of Deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). J Gen Virol 86 : 3419–3424 doi:10.1099/vir.0.81401-0
17. ShenM, YangX, Cox-FosterD, CuiL (2005) The role of varroa mites in infections of Kashmir bee virus (KBV) and deformed wing virus (DWV) in honey bees. Virology 342 : 141–149 doi:10.1016/j.virol.2005.07.012
18. ChenY, EvansJ, FeldlauferM (2006) Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. J Invertebr Pathol 92 : 152–159 doi:10.1016/j.jip.2006.03.010
19. MerklingSH, van RijRP (2013) Beyond RNAi: antiviral defense strategies in Drosophila and mosquito. J Insect Physiol 59 : 159–170 doi:10.1016/j.jinsphys.2012.07.004
20. SabinLR, HannaSL, CherryS (2010) Innate antiviral immunity in Drosophila. Curr Opin Immunol 22 : 4–9 doi:10.1016/j.coi.2010.01.007
21. KempC, ImlerJ-L (2009) Antiviral immunity in drosophila. Curr Opin Immunol 21 : 3–9 doi:10.1016/j.coi.2009.01.007
22. Brun N, Plus N (1980) The viruses of Drosophila. In: Ashburner and M, Wright TRF, editors. The genetics and Biology of Drosophila. New York: Academic Press. pp. 625–702.
23. HabayebMS, EkengrenSK, HultmarkD (2006) Nora virus, a persistent virus in Drosophila, defines a new picorna-like virus family. J Gen Virol 87 : 3045–3051 doi:10.1099/vir.0.81997-0
24. HabayebMS, CanteraR, CasanovaG, EkströmJ-O, AlbrightS, et al. (2009) The Drosophila Nora virus is an enteric virus, transmitted via feces. J Invertebr Pathol 101 : 29–33 doi:10.1016/j.jip.2009.02.003
25. JoussetFX, PlusN (1975) [Study of the vertical transmission and horizontal transmission of “Drosophila melanogaster” and “Drosophila immigrans” picornavirus (author's transl)]. Ann Microbiol (Paris) 126 : 231–249.
26. Gomariz-ZilberE, PorasM, Thomas-OrillardM (1995) Drosophila C virus: experimental study of infectious yields and underlying pathology in Drosophila melanogaster laboratory populations. J Invertebr Pathol 65 : 243–247 doi:10.1006/jipa.1995.1037
27. FilipeD, Thomas-orillardM (1998) Experimental study of a Drosophila melanogaster laboratory population infected by food contamination. Endocytobiosis Cell Res 12 : 163–176.
28. Roxström-LindquistK, TereniusO, FayeI (2004) Parasite-specific immune response in adult Drosophila melanogaster: a genomic study. EMBO Rep 5 : 207–212 doi:10.1038/sj.embor.7400073
29. XuJ, HopkinsK, SabinL, YasunagaA, SubramanianH, et al. (2013) ERK signaling couples nutrient status to antiviral defense in the insect gut. Proc Natl Acad Sci U S A 110 : 15025–15030 doi:10.1073/pnas.1303193110
30. Van RijRP, SalehM, BerryB, FooC, HoukA, et al. (2006) The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev 20 : 2985–2995 doi:10.1101/gad.1482006
31. Galiana-ArnouxD, DostertC, SchneemannA, HoffmannJA, ImlerJ-L (2006) Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nat Immunol 7 : 590–597 doi:10.1038/ni1335
32. WangX-H, AliyariR, LiW-X, LiH-W, KimK, et al. (2006) RNA interference directs innate immunity against viruses in adult Drosophila. Science 312 : 452–454 doi:10.1126/science.1125694
33. ZambonRA, VakhariaVN, WuLP (2006) RNAi is an antiviral immune response against a dsRNA virus in Drosophila melanogaster. Cell Microbiol 8 : 880–889 doi:10.1111/j.1462-5822.2006.00688.x
34. BronkhorstAW, van CleefKWR, VodovarN, InceIA, BlancH, et al. (2012) The DNA virus Invertebrate iridescent virus 6 is a target of the Drosophila RNAi machinery. Proc Natl Acad Sci U S A 109: E3604–13 doi:10.1073/pnas.1207213109
35. KempC, MuellerS, GotoA, BarbierV, ParoS, et al. (2013) Broad RNA interference-mediated antiviral immunity and virus-specific inducible responses in Drosophila. J Immunol 190 : 650–658 doi:10.4049/jimmunol.1102486
36. LiH, LiWX, DingSW (2002) Induction and suppression of RNA silencing by an animal virus. Science 296 : 1319–1321 doi:10.1126/science.1070948
37. TeixeiraL, FerreiraA, AshburnerM (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6: e2 doi:10.1371/journal.pbio.1000002
38. HedgesLM, BrownlieJC, O'NeillSL, JohnsonKN (2008) Wolbachia and virus protection in insects. Science 322 : 702 doi:10.1126/science.1162418
39. RancèsE, YeYH, WoolfitM, McGraw Ea, O'NeillSL (2012) The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog 8: e1002548 doi:10.1371/journal.ppat.1002548
40. GlaserRL, MeolaMA (2010) The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE 5: e11977 doi:10.1371/journal.pone.0011977
41. DostertC, JouanguyE, IrvingP, TroxlerL, Galiana-ArnouxD, et al. (2005) The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat Immunol 6 : 946–953 doi:10.1038/ni1237
42. CostaA, JanE, SarnowP, SchneiderD (2009) The Imd pathway is involved in antiviral immune responses in Drosophila. PLoS ONE 4: e7436 doi:10.1371/journal.pone.0007436
43. AvadhanulaV, WeasnerBP, HardyGG, KumarJP, HardyRW (2009) A novel system for the launch of alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS Pathog 5: e1000582 doi:10.1371/journal.ppat.1000582
44. SabatierL, JouanguyE, DostertC, ZacharyD, DimarcqJ-L, et al. (2003) Pherokine-2 and -3. Eur J Biochem 270 : 3398–3407 doi:10.1046/j.1432-1033.2003.03725.x
45. IpYT, ReachM, EngstromY, KadalayilL, CaiH, et al. (1993) Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell 75 : 753–763.
46. MengX, KhanujaBS, IpYT (1999) Toll receptor-mediated Drosophila immune response requires Dif, an NF-kappaB factor. Genes Dev 13 : 792–797.
47. ManfruelliP, ReichhartJM, StewardR, HoffmannJA, LemaitreB (1999) A mosaic analysis in Drosophila fat body cells of the control of antimicrobial peptide genes by the Rel proteins Dorsal and DIF. EMBO J 18 : 3380–3391 doi:10.1093/emboj/18.12.3380
48. ZambonRA, NandakumarM, VakhariaVN, WuLP (2005) The Toll pathway is important for an antiviral response in Drosophila. Proc Natl Acad Sci U S A 102 : 7257–7262 doi:10.1073/pnas.0409181102
49. NazziF, BrownSP, AnnosciaD, Del PiccoloF, Di PriscoG, et al. (2012) Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog 8: e1002735 doi:10.1371/journal.ppat.1002735
50. XiZ, RamirezJL, DimopoulosG (2008) The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog 4: e1000098 doi:10.1371/journal.ppat.1000098
51. JohnsonKN, ChristianPD (1998) The novel genome organization of the insect picorna-like virus Drosophila C virus suggests this virus belongs to a previously undescribed virus family. J Gen Virol 79 (Pt 1) 191–203.
52. RyderE, BlowsF, AshburnerM, Bautista-LlacerR, CoulsonD, et al. (2004) The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics 167 : 797–813 doi:10.1534/genetics.104.026658
53. RutschmannS, JungAC, HetruC, ReichhartJ-M, HoffmannJA, et al. (2000) The Rel Protein DIF Mediates the Antifungal but Not the Antibacterial Host Defense in Drosophila. Immunity 12 : 569–580 doi:10.1016/S1074-7613(00)80208-3
54. RutschmannS, KilincA, FerrandonD (2002) Cutting edge: the toll pathway is required for resistance to gram-positive bacterial infections in Drosophila. J Immunol 168 : 1542–1546.
55. LigoxygakisP, PelteN, HoffmannJA, ReichhartJ-M (2002) Activation of Drosophila Toll during fungal infection by a blood serine protease. Science 297 : 114–116 doi:10.1126/science.1072391
56. GobertV, GottarM, MatskevichAA, RutschmannS, RoyetJ, et al. (2003) Dual activation of the Drosophila toll pathway by two pattern recognition receptors. Science 302 : 2126–2130 doi:10.1126/science.1085432
57. MoreiraLA, Iturbe-OrmaetxeI, JefferyJA, LuG, PykeAT, et al. (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139 : 1268–1278 doi:10.1016/j.cell.2009.11.042
58. PanX, ZhouG, WuJ, BianG, LuP, et al. (2012) Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A 109: E23–31 doi:10.1073/pnas.1116932108
59. RancèsE, JohnsonTK, PopoviciJ, Iturbe-OrmaetxeI, ZakirT, et al. (2013) The toll and Imd pathways are not required for wolbachia-mediated dengue virus interference. J Virol 87 : 11945–11949 doi:10.1128/JVI.01522-13
60. TateJ, LiljasL, ScottiP, ChristianP, LinT, et al. (1999) The crystal structure of cricket paralysis virus: the first view of a new virus family. Nat Struct Biol 6 : 765–774 doi:10.1038/11543
61. ScottiPD, DearingS, MossopDW (1983) Flock House virus: a nodavirus isolated from Costelytra zealandica (White) (Coleoptera: Scarabaeidae). Arch Virol 75 : 181–189 doi:10.1007/BF01315272
62. WhalenAM, StewardR (1993) Dissociation of the dorsal-cactus complex and phosphorylation of the dorsal protein correlate with the nuclear localization of dorsal. J Cell Biol 123 : 523–534.
63. MichelT, ReichhartJM, HoffmannJA, RoyetJ (2001) Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 414 : 756–759 doi:10.1038/414756a
64. ReichhartJM, GeorgelP, MeisterM, LemaitreB, KapplerC, et al. (1993) Expression and nuclear translocation of the rel/NF-kappa B-related morphogen dorsal during the immune response of Drosophila. C R Acad Sci III 316 : 1218–1224.
65. GrossI, GeorgelP, Oertel-BuchheitP, SchnarrM, ReichhartJM (1999) Dorsal-B, a splice variant of the Drosophila factor Dorsal, is a novel Rel/NF-kappaB transcriptional activator. Gene 228 : 233–242.
66. MatovaN, Anderson KV (2006) Rel/NF-kappaB double mutants reveal that cellular immunity is central to Drosophila host defense. Proc Natl Acad Sci U S A 103 : 16424–16429 doi:10.1073/pnas.0605721103
67. HanZS, IpYT (1999) Interaction and specificity of Rel-related proteins in regulating Drosophila immunity gene expression. J Biol Chem 274 : 21355–21361.
68. TanjiT, YunE-Y, IpYT (2010) Heterodimers of NF-kappaB transcription factors DIF and Relish regulate antimicrobial peptide genes in Drosophila. Proc Natl Acad Sci U S A 107 : 14715–14720 doi:10.1073/pnas.1009473107
69. MatovaN, AndersonKV (2010) Drosophila Rel proteins are central regulators of a robust, multi-organ immune network. J Cell Sci 123 : 627–633 doi:10.1242/jcs.060731
70. Nüsslein-VolhardC, Lohs-SchardinM, SanderK, CremerC (1980) A dorso-ventral shift of embryonic primordia in a new maternal-effect mutant of Drosophila. Nature 283 : 474–476 doi:10.1038/283474a0
71. RoseD, ZhuX, KoseH, HoangB, ChoJ, et al. (1997) Toll, a muscle cell surface molecule, locally inhibits synaptic initiation of the RP3 motoneuron growth cone in Drosophila. Development 124 : 1561–1571.
72. HalfonMS, HashimotoC, KeshishianH (1995) The Drosophila toll gene functions zygotically and is necessary for proper motoneuron and muscle development. Dev Biol 169 : 151–167 doi:10.1006/dbio.1995.1134
73. HalfonMS, KeshishianH (1998) The Toll pathway is required in the epidermis for muscle development in the Drosophila embryo. Dev Biol 199 : 164–174 doi:10.1006/dbio.1998.8915
74. SutcliffeB, ForeroMG, ZhuB, RobinsonIM, HidalgoA (2013) Neuron-type specific functions of DNT1, DNT2 and Spz at the Drosophila neuromuscular junction. PLoS One 8: e75902 doi:10.1371/journal.pone.0075902
75. HeckscherES, FetterRD, MarekKW, AlbinSD, DavisGW (2007) NF-kappaB, IkappaB, and IRAK control glutamate receptor density at the Drosophila NMJ. Neuron 55 : 859–873 doi:10.1016/j.neuron.2007.08.005
76. QiuP, PanPC, GovindS (1998) A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development 125 : 1909–1920.
77. GottarM, GobertV, Matskevich Aa, ReichhartJ-M, WangC, et al. (2006) Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell 127 : 1425–1437 doi:10.1016/j.cell.2006.10.046
78. El ChamyL, LeclercV, CaldelariI, ReichhartJ-M (2008) Sensing of “danger signals” and pathogen-associated molecular patterns defines binary signaling pathways “upstream” of Toll. Nat Immunol 9 : 1165–1170 doi:10.1038/ni.1643
79. ParisiF, StefanatosRK, StrathdeeK, YuY, VidalM (2014) Transformed epithelia trigger non-tissue-autonomous tumor suppressor response by adipocytes via activation of Toll and Eiger/TNF signaling. Cell Rep 6 : 855–867 doi:10.1016/j.celrep.2014.01.039
80. MingM, ObataF, KuranagaE, MiuraM (2014) Persephone/Spätzle pathogen sensors mediate the activation of Toll receptor signaling in response to endogenous danger signals in apoptosis-deficient Drosophila. J Biol Chem 289 : 7558–7568 doi:10.1074/jbc.M113.543884
81. DeddoucheS, MattN, BuddA, MuellerS, KempC, et al. (2008) The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in drosophila. Nat Immunol 9 : 1425–1432 doi:10.1038/ni.1664
82. NakamotoM, MoyRH, XuJ, BambinaS, YasunagaA, et al. (2012) Virus recognition by Toll-7 activates antiviral autophagy in Drosophila. Immunity 36 : 658–667 doi:10.1016/j.immuni.2012.03.003
83. HabayebMS, EkströmJ-O, HultmarkD (2009) Nora virus persistent infections are not affected by the RNAi machinery. PLoS One 4: e5731 doi:10.1371/journal.pone.0005731
84. Di PasqualeG, ChioriniJA (2006) AAV transcytosis through barrier epithelia and endothelium. Mol Ther 13 : 506–516 doi:10.1016/j.ymthe.2005.11.007
85. OuzilouL, CaliotE, PelletierI, PrévostM-C, PringaultE, et al. (2002) Poliovirus transcytosis through M-like cells. J Gen Virol 83 : 2177–2182.
86. WangY, Gosselin GrenetAS, CastelliI, CermenatiG, RavallecM, et al. (2013) Densovirus crosses the insect midgut by transcytosis and disturbs the epithelial barrier function. J Virol 87 : 12380–12391 doi:10.1128/JVI.01396-13
87. LiuB, BehuraSK, ClemRJ, SchneemannA, BecnelJ, et al. (2013) P53-mediated rapid induction of apoptosis conveys resistance to viral infection in Drosophila melanogaster. PLoS Pathog 9: e1003137 doi:10.1371/journal.ppat.1003137
88. VaidyanathanR, ScottTW (2006) Apoptosis in mosquito midgut epithelia associated with West Nile virus infection. Apoptosis 11 : 1643–1651 doi:10.1007/s10495-006-8783-y
89. MorganNS, SkovronskyDM, Artavanis-TsakonasS, MoosekerMS (1994) The molecular cloning and characterization of Drosophila melanogaster myosin-IA and myosin-IB. J Mol Biol 239 : 347–356 doi:10.1006/jmbi.1994.1376
90. AndersonKV, Nüsslein-VolhardC (1984) Information for the dorsal–ventral pattern of the Drosophila embryo is stored as maternal mRNA. Nature 311 : 223–227 doi:10.1038/311223a0
91. AndersonKV, JürgensG, Nüsslein-VolhardC (1985) Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 42 : 779–789.
92. HechtPM, AndersonKV (1993) Genetic characterization of tube and pelle, genes required for signaling between Toll and dorsal in the specification of the dorsal-ventral pattern of the Drosophila embryo. Genetics 135 : 405–417.
93. Nüsslein-Volhard C (1979) Maternal effect mutations that alter the saptial coordinates of Drosophila melanogaster. In: Subtelny S, Konigsberg IR, editors. Determinats of Spatial Organization. New York: Academic Press. pp. 185–211.
94. RynesJ, DonohoeCD, FrommoltP, BrodesserS, JindraM, et al. (2012) Activating transcription factor 3 regulates immune and metabolic homeostasis. Mol Cell Biol 32 : 3949–3962 doi:10.1128/MCB.00429-12
95. OsterwalderT, YoonKS, WhiteBH, KeshishianH (2001) A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A 98 : 12596–12601 doi:10.1073/pnas.221303298
96. BuchonN, OsmanD, DavidFPA, FangHY, BoqueteJ-P, et al. (2013) Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep 3 : 1725–1738 doi:10.1016/j.celrep.2013.04.001
97. O'BrienLE, SolimanSS, LiX, BilderD (2011) Altered modes of stem cell division drive adaptive intestinal growth. Cell 147 : 603–614 doi:10.1016/j.cell.2011.08.048
98. ShiaAKH, GlittenbergM, ThompsonG, WeberAN, ReichhartJ-M, et al. (2009) Toll-dependent antimicrobial responses in Drosophila larval fat body require Spätzle secreted by haemocytes. J Cell Sci 122 : 4505–4515 doi:10.1242/jcs.049155
99. NiJ-Q, ZhouR, CzechB, LiuL-P, HolderbaumL, et al. (2011) A genome-scale shRNA resource for transgenic RNAi in Drosophila. Nat Methods 8 : 405–407 doi:10.1038/nmeth.1592
100. ChrostekE, MarialvaMSP, EstevesSS, WeinertLA, MartinezJ, et al. (2013) Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenomic analysis. PLoS Genet 9: e1003896 doi:10.1371/journal.pgen.1003896
101. ScottiPD (1977) End-point dilution and plaque assay methods for titration of cricket paralysis virus in cultured Drosophila cells. J Gen Virol 35 : 393–396.
102. SchindelinJ, Arganda-CarrerasI, FriseE, KaynigV, LongairM, et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9 : 676–682 doi:10.1038/nmeth.2019
103. ChuDT, KlymkowskyMW (1989) The appearance of acetylated alpha-tubulin during early development and cellular differentiation in Xenopus. Dev Biol 136 : 104–117.
104. PfafflMW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research 29: e45.
105. SharonG, SegalD, RingoJM, HefetzA, Zilber-RosenbergI, et al. (2010) Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci U S A 107 : 20051–20056 doi:10.1073/pnas.1009906107
106. RyuJ-H, KimS-H, LeeH-Y, BaiJY, NamY-D, et al. (2008) Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 319 : 777–782 doi:10.1126/science.1149357
107. R Development Core Team RFFSC (2008) R: A Language and Environment for Statistical Computing. Vienna Austria R Found Stat Comput 1 : 2673.
108. Zar JH (1999) Biostatistical Analysis. Prentice Hall, New Jersey, 663 pp.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Selective Susceptibility of Human Skin Antigen Presenting Cells to Productive Dengue Virus InfectionČlánek P47 Mice Are Compromised in Expansion and Activation of CD8 T Cells and Susceptible to InfectionČlánek Molecular Evolution of Broadly Neutralizing Llama Antibodies to the CD4-Binding Site of HIV-1
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 12- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Microbial Programming of Systemic Innate Immunity and Resistance to Infection
- Unique Features of HIV-1 Spread through T Cell Virological Synapses
- Measles Immune Suppression: Functional Impairment or Numbers Game?
- Cellular Mechanisms of Alpha Herpesvirus Egress: Live Cell Fluorescence Microscopy of Pseudorabies Virus Exocytosis
- Rubella Virus: First Calcium-Requiring Viral Fusion Protein
- Plasma Membrane-Located Purine Nucleotide Transport Proteins Are Key Components for Host Exploitation by Microsporidian Intracellular Parasites
- Selective Susceptibility of Human Skin Antigen Presenting Cells to Productive Dengue Virus Infection
- Loss of Dynamin-Related Protein 2B Reveals Separation of Innate Immune Signaling Pathways
- Intraspecies Competition for Niches in the Distal Gut Dictate Transmission during Persistent Infection
- Unveiling the Intracellular Survival Gene Kit of Trypanosomatid Parasites
- Extreme Divergence of Tropism for the Stem-Cell-Niche in the Testis
- HTLV-1 Tax-Mediated Inhibition of FOXO3a Activity Is Critical for the Persistence of Terminally Differentiated CD4 T Cells
- P47 Mice Are Compromised in Expansion and Activation of CD8 T Cells and Susceptible to Infection
- Hypercytotoxicity and Rapid Loss of NKp44 Innate Lymphoid Cells during Acute SIV Infection
- Molecular Evolution of Broadly Neutralizing Llama Antibodies to the CD4-Binding Site of HIV-1
- Crystal Structure of Calcium Binding Protein-5 from and Its Involvement in Initiation of Phagocytosis of Human Erythrocytes
- Chronic Parasitic Infection Maintains High Frequencies of Short-Lived Ly6CCD4 Effector T Cells That Are Required for Protection against Re-infection
- Specific Dysregulation of IFNγ Production by Natural Killer Cells Confers Susceptibility to Viral Infection
- HSV-2-Driven Increase in the Expression of αβ Correlates with Increased Susceptibility to Vaginal SHIV Infection
- Murine Anti-vaccinia Virus D8 Antibodies Target Different Epitopes and Differ in Their Ability to Block D8 Binding to CS-E
- Brothers in Arms: Th17 and Treg Responses in Immunity
- Granulocytes Impose a Tight Bottleneck upon the Gut Luminal Pathogen Population during Typhimurium Colitis
- A Negative Feedback Modulator of Antigen Processing Evolved from a Frameshift in the Cowpox Virus Genome
- Discovery of Replicating Circular RNAs by RNA-Seq and Computational Algorithms
- The Non-receptor Tyrosine Kinase Tec Controls Assembly and Activity of the Noncanonical Caspase-8 Inflammasome
- Targeted Changes of the Cell Wall Proteome Influence Ability to Form Single- and Multi-strain Biofilms
- Apoplastic Venom Allergen-like Proteins of Cyst Nematodes Modulate the Activation of Basal Plant Innate Immunity by Cell Surface Receptors
- The Toll-Dorsal Pathway Is Required for Resistance to Viral Oral Infection in
- Anti-α4 Antibody Treatment Blocks Virus Traffic to the Brain and Gut Early, and Stabilizes CNS Injury Late in Infection
- Initiation of ART during Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation
- Microbial Urease in Health and Disease
- Emergence of MERS-CoV in the Middle East: Origins, Transmission, Treatment, and Perspectives
- Blocking Junctional Adhesion Molecule C Enhances Dendritic Cell Migration and Boosts the Immune Responses against
- IL-28B is a Key Regulator of B- and T-Cell Vaccine Responses against Influenza
- A Natural Genetic Variant of Granzyme B Confers Lethality to a Common Viral Infection
- Neutral Sphingomyelinase in Physiological and Measles Virus Induced T Cell Suppression
- Differential PfEMP1 Expression Is Associated with Cerebral Malaria Pathology
- The Role of the NADPH Oxidase NOX2 in Prion Pathogenesis
- Rapid Evolution of Virus Sequences in Intrinsically Disordered Protein Regions
- The Central Role of cAMP in Regulating Merozoite Invasion of Human Erythrocytes
- Expression of Suppressor of Cytokine Signaling 1 (SOCS1) Impairs Viral Clearance and Exacerbates Lung Injury during Influenza Infection
- Cellular Oxidative Stress Response Controls the Antiviral and Apoptotic Programs in Dengue Virus-Infected Dendritic Cells
- SUMOylation by the E3 Ligase TbSIZ1/PIAS1 Positively Regulates VSG Expression in
- Monocyte Recruitment to the Dermis and Differentiation to Dendritic Cells Increases the Targets for Dengue Virus Replication
- Oral Streptococci Utilize a Siglec-Like Domain of Serine-Rich Repeat Adhesins to Preferentially Target Platelet Sialoglycans in Human Blood
- SV40 Utilizes ATM Kinase Activity to Prevent Non-homologous End Joining of Broken Viral DNA Replication Products
- Amphipathic α-Helices in Apolipoproteins Are Crucial to the Formation of Infectious Hepatitis C Virus Particles
- Proteomic Analysis of the Acidocalcisome, an Organelle Conserved from Bacteria to Human Cells
- Experimental Cerebral Malaria Pathogenesis—Hemodynamics at the Blood Brain Barrier
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Plasma Membrane-Located Purine Nucleotide Transport Proteins Are Key Components for Host Exploitation by Microsporidian Intracellular Parasites
- Rubella Virus: First Calcium-Requiring Viral Fusion Protein
- Emergence of MERS-CoV in the Middle East: Origins, Transmission, Treatment, and Perspectives
- Unique Features of HIV-1 Spread through T Cell Virological Synapses
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



