-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Flow Cytometric Phenotyping and Analysis of T Regulatory Cells in Multiple Myeloma Patients
Fenotypizace a kvantifikace T regulačních lymfocytů u pacientů s mnohočetným myelomem pomocí průtokové cytometrie
Mnohočetný myelom (MM) je onemocnění plazmatických buněk (PC), které bývá často spojeno s poruchami imunity. Fenotypizace a stanovení počtu regulačních T lymfocytů (Tregs) pomocí průtokové cytometrie může být využito k monitorování stavu imunity u myelomových pacientů. Charakterizace funkčního stavu Tregs pomocí proliferačních či inhibičních testů pak může odhalit jejich možnou poruchu. V naší studii bylo zjištěno, že u pacientů s MM jsou počty Tregs zvýšeny oproti zdravým kontrolám, což u těchto pacientů svědčí o deregulaci imunity.
Klíčová slova:
Treg buňky – flow cytometrie – mnohočetný myelom – thalidomid
Tato práce byla podpořena granty MŠMT ČR (MSM0021622434, LC06027), MZd ČR (IGA grants NS10406, NS10408) a GAČR GAP304/10/1395.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do bi omedicínských časopisů.
Authors: Muthu Raja K. R. 1,2; L. Kovářová 1,3; J. Štossová 1; R. Hájek 1,3,4
Authors place of work: Babak Myeloma Group, Department of Pathological Physiology, Faculty of Medicine, Masaryk University, Brno, Czech Republic 1; Department of Molecular and Cellular Biology, Faculty of Science, Masaryk University, Brno, Czech Republic 2; Laboratory of Experimental Hematology and Cell Immunotherapy, Department of Clinical Hematology, University Hospital Brno, Czech Republic 3; Department of Internal Medicine – Hematooncology, University Hospital Brno, Czech Republic 4
Published in the journal: Klin Onkol 2011; 24(Supplementum 1): 30-33
Summary
Multiple myeloma (MM) is a plasma cell (PC) disorder and associated with immune impairments. Flow cytometry based phenotyping and quantification of regulatory T cells (Tregs) enable to monitor the immune status of myeloma patients. Apart from enumeration of Tregs, functional characterization using proliferation or suppression assay adds key value in demonstrating the functional value of Tregs. Our study revealed that in MM patients Tregs are elevated compared to healthy subjects, which demonstrate the immune deregulation in MM.
Key words:
Tregs – flow cytometry – multiple myeloma – thalidomide
This study was supported by grants of the Czech Ministry of Education, Youth and Sports (MSM0021622434, LC06027), Ministry of Health (IGA grants NS10406, NS10408), and GACR GAP304/10/1395.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.Introduction
Multiple myeloma (MM) is a malignant plasma cell (PC) disorder characterized by higher (≥ 10%) PC infiltrations and ≥ 30 g/L of monoclonal protein (M-protein), whereas monoclonal gammopathy of undetermined significance (MGUS) characterized by < 10% of PC infiltrations and < 30 g/L of M-protein [1,2]. There are evidences available in MM for impaired T cells counts and functional abnormalities [3]. Recently, a study proved increased number of regulatory T cells (Tregs) in cancer patients [4]. Tregs plays active role in establishing and maintaining immunological unresponsiveness to self antigens and negative control of various immune responses to non-self antigens [5]. Regulatory function for Tregs is provided by a master molecule FoxP3. At present, several studies proved that Tregs were expanded both in hematological malignancies and solid tumors [6,7].
Subtypes of T regulatory cells:
Natural Tregs – Arise from thymus and disseminate to periphery; these cells constitute about 10%–15% of CD4 cells. Destruction in the development or function of natural Tregs leads to autoimmune diseases [8].
Tr1 regulatory cells – Induced from the peripheral naïve T cells in the presence of IL-10. These cells lack FoxP3 expression but secrete IL-10 and TGF (transforming growth factor)-β [9].
Th3 cells – Induced from the peripheral naïve T cells in the presence of TGF-β. Secrete mostly TGF-β for suppression. Rare Th3 cells express FoxP3 molecule due to induction by TGF-β [10].
Double negative (DN) Tregs – In mice and humans, these cells constitute about 1–3% and 1%, respectively. DN Tregs inhibit T cell activation and proliferation in antigen-specific manner [11].
γδ T cells – Suppress naïve and effector T cell responses and inhibit maturation and function of dendritic cells [12].
NKT (natural killer T) regulatory cells – CD1d dependent/restricted type II NKT cells are able to suppress tumor immune surveillance, but type I NKT cells lack suppressive function [13].
Functions of T regulatory cells:
Release of inhibitory cytokines – Mainly cytokines such as IL-10, TGF-β and IL-35 are secreted by Tregs that are involved in inhibitory function. Peptide inhibitor targeted against the surface TGF-β on Tregs abrogates their function and enhances the anti-tumour response [14].
Cytotoxicity – Perforin/granzyme pathway is well-known to be associated with CD8 T cells and NK cells for destruction of intracellular pathogens and tumour cells. Recent studies have shown Tregs also use perforin/granzyme pathway [15,16].
Inhibition of antigen presenting cells (APCs) – Expression of cytotoxic T lymphocyte antigen-4 (CTLA-4) under the control of FoxP3 by Tregs facilitates the interaction with APCs co-stimulatory molecules CD80 and CD86 and induces suppression of T cell activation [17].
Recommended Methodology for Identification and Characterization of T Regulatory Cells
Flow Cytometry Method for Identification of T Regulatory Cells
Flow cytometry based identification of Tregs is a feasible method. Globally, a simple three-color flow cytometric analysis including CD4, CD25 and FoxP3 is able to characterize and quantify the Tregs in MM. To characterize the Tregs more precisely we use four-color cytometry with the inclusion of additional marker CD127 and the protocol is summarized here. One-two millions of erythrocytes lysed peripheral blood (PB) cells are labeled with the following fluorochrome conjugated monoclonal antibodies: phycoerythrin-cyanin 7 (PE-Cy7)-CD4, allophycocyanin (APC)--CD25 and phycoerythrin (PE)-CD127, and incubated at 4°C for 20–30 min (all monoclonal antibodies are obtained from BD Biosciences). Then, cells are fixed and permeabilized according to eBioscience recommendations (eBioscience, San Diego, CA), and finally, cells are labeled with FoxP3 antibody conjugated with fluorescein isothiocyanate (FITC) from eBioscience and incubated at 4°C for 30–60 min. All prepared samples are measured on BD FACSCanto IITM and approximately 300–400 thousands of events are acquired to enumerate the Tregs. For negative control, FITC conjugated isotype antibody is used. We usually identify and quantify Tregs by the phenotype CD4+CD25hi+FoxP3+ along with presence or absence of CD127 (Fig. 1). Inclusion of CD127 in the analysis will add value in defining the Tregs because these cells usually have dim/negative expression for CD127.
Fig. 1. Phenotypic feature of T regulatory cells. These two dot plots represent the typical phenotypic nature of Tregs (CD4+CD25hi+FoxP3+). Dot plot in the left (MGUS) demonstrates decrease in the frequency of Tregs when compared to MM dot plot in the right. 
Methods for Functional Characterization of T Regulatory Cells
Two methods such as 5, 6-carboxyfluorescein-diacetate succinimidyl-ester (CFSE) and 3H-thymidine could be used for assessing the suppressive function of Tregs according to literature. The assay includes the separation of Tregs (CD4+CD25hi+) and responder cells (CD4+CD25–) either by magnetic bead separation or flow cytometry based separation. Tregs are mixed in different proportions to stimulated responder cells with anti-CD3/anti-CD28 beads or irradiated allogenic peripheral blood mononuclear cells (PBMNCs) along with CFSE labeling. When there will be proliferation of responder cells CFSE intensity will decrease, the duration of assay period is 4–6 days (Fig. 2) [21]. In 3H-thymidine assay after incubation of Tregs and stimulated responder cells together for 3 days. The proliferation of responder cells is measured by incorporation of 3H-thymidine for the last 18 hours during the incubation period [19,20].
Our Study Experience of T Regulatory Cells in Multiple Myeloma Patients
We analyzed Tregs in PB of 69 monoclonal gammopathy (MG) patients including MGUS - (11/69), SMM - (5/69), newly diagnosed MM - (40/69) and relapsed MM - (13/69). For comparison, 12 healthy volunteers PB was also analyzed (Tab. 1). A cohort of 20 newly diagnosed MM patients were followed-up to determine the PB Tregs number after 4 treatment cycles with thalidomide plus cyclophosphamide and dexamethasone (CTD). To analyze the difference between two groups Mann-Whitney U test was used and P value of ≤ 0.05 was considered as statistically significant.
Tab. 1. Comparison of peripheral blood Tregs frequencies between healthy volunteers and MM patients. 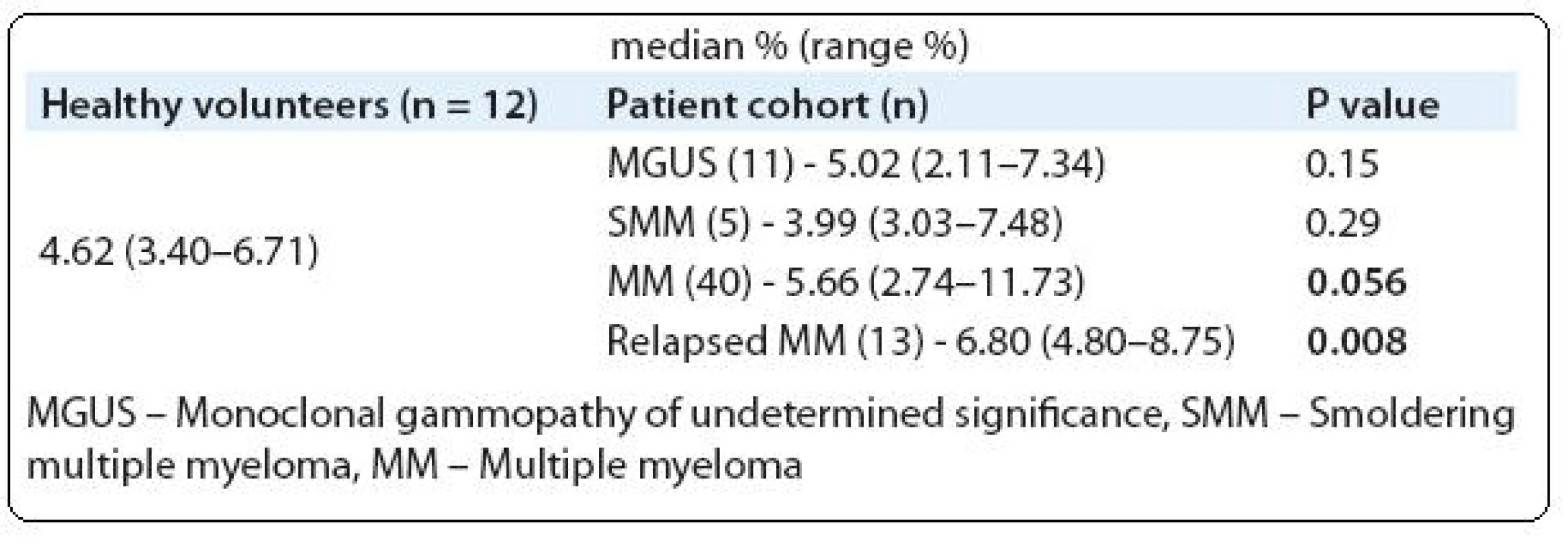
Results and Discussion
In consistent with other studies, our study also showed PB Tregs were increased in MM patients (Tab. 1) [18,19]. In contrast to Beyer et al and Feyler et al studies we did not observe any significant expansion of PB Tregs in MGUS and SMM cohorts compared to healthy volunteers [18,19]. Prabhala et al study showed significantly reduced FoxP3 expressing CD4 T cells in MGUS and MM patients which is contrasting to our observation and other studies [18–20]. This conflicting result might be due to the use of different identification strategy. For instance, Prabhala et al and Brimnes et al studies used only CD4 and FoxP3 molecules for identification [20,22]. British study characterized and quantified the Tregs as CD4+CD25hi+FoxP3+, which is the globally accepted phenotypic feature of Tregs [19]. In contrast, German study identified Tregs as CD4+CD25hi+ [18]. This identification will give the information about 80–90% of Tregs but with out combination of FoxP3 one might not completely characterize Tregs. Gupta et al used CD127 along with CD25 to identify Tregs [21].
Tregs in MM patients were proved to be functionally active as similar to healthy volunteers Tregs [18,19,22]. Exclusively, Prabhala et al study showed MM patients Tregs failed to suppress the proliferation of responder cells when compared to healthy subjects Tregs [20]. This contrasting result by Prabhala et al study might be due to the use of PBMNCs as responder cells [20]. In concordance with in vitro findings in MM, an in vivo study showed, after allogenic stem cell transplantation the donor-derived Tregs reconstituted in the bone marrow (BM) were functional and also enhanced the survival of transplant without graft versus host disease [23]. As similarly to MM, dysfunctional and increased number of Tregs were also documented in various hematological malignancies including B-cell chronic lymphocytic leukemia (B-CLL), acute myeloid leukemia and non-Hodgkins lymphoma [7,24,25].
Our observation showed a trend of increase in Tregs number (Tab. 2) after treatment with thalidomide combination (CTD), but in B-CLL patient’s substantial decrease in Tregs number was reported after thalidomide plus fludarabine treatment [26]. The possible reason behind the observation of increased Tregs after thalidomide is IL-6 has the ability to decrease the number of Tregs, which was proved in murine models. Well known functions of thalidomide in MM are downregulation of various adhesion molecules on PCs and cytokine molecules such as IL-6, TNF-α and RANKL [27]. From this point we could able to clarify that after thalidomide treatment the level of IL-6 reduces and as a cascade the Tregs increase in MM patients. Unprecedently, our data showed positive association between Tregs and BMPC infiltrations (r = 0.25; P = 0.034).
Tab. 2. Assessment of Tregs frequencies between pre treatment versus post treatment cycles (CTD). 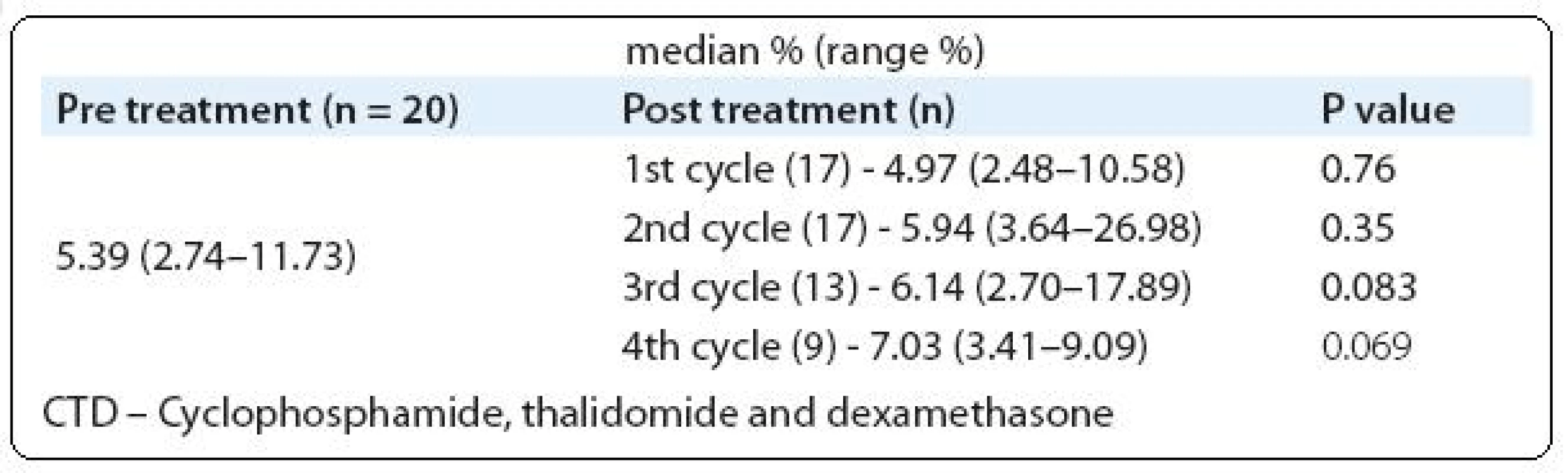
In summary flow cytometry based analysis of Tregs is a useful method which facilitates to understand the immune status in MM patients. This technique is also feasible to monitor the MM patients before and after treatment to screen the changes of regulatory and immune cells. Using flow cytometry, several studies and our observation proved that Tregs were elevated in MM patients. This observation should be taken into consideration to improve the immune status in myeloma patients by following different treatment approaches.
Prof. MUDr. Roman Hájek, CSc.
Babak Myeloma Group
Department of Pathological Physiology
Faculty of Medicine
Masaryk University
Kamenice 5
625 00 Brno
Czech Republic
e-mail: r.hajek@fnbrno.cz
Zdroje
1. Kyle RA, Rajkumar SV. Criteria for diagnosis staging risk stratification and response assessment of multiple myeloma. Leukemia 2009; 23(1): 3–9.
2. Raja KR, Kovarova L, Hajek R. Review of phenotypic markers used in flow cytometric analysis of MGUS and MM and applicability of flow cytometry in other plasma cell disorders. Br J Haematol 2010; 149(3): 334–351.
3. Mills KH, Cawley JC. Abnormal monoclonal antibody defined helper/suppressor T-cell subpopulations in multiple myeloma: relationship to treatment and clinical stage. Br J Haematol 1983; 53(2): 271–275.
4. Wolf AM, Wolf D, Steurer M et al. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 2003; 9(2): 606–612.
5. Fehérvari Z, Sakaguchi S. CD4+ Tregs and immune control. J Clin Invest 2004; 114(9): 1209–1217.
6. Bates GJ, Fox SB, Han C et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 2006; 24(34): 5373–5380.
7. Beyer M, Kochanek M, Darabi K et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood 2005; 106(6): 2018–2025.
8. Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol 2003; 15(4): 430–435.
9. Vieira PL, Christensen JR, Minaee S et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol 2004; 172(10): 5986–5993.
10. Chen W, Jin W, Hardegen N et al. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003; 198(12): 1875–1886.
11. Fischer K, Voelkl S, Heymann J et al. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4-CD8 - double-negative regulatory T cells. Blood 2005; 105(7): 2828–2835.
12. Peng G, Wang HY, Peng W et al. Tumor-infiltrating gamma delta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity 2007; 27(2): 334–348.
13. Wang RF. CD8+ regulatory T cells, their suppressive mechanisms, and regulation in cancer. Hum Immunol 2008; 69(11): 811–814.
14. Gil-Guerrero L, Dotor J, Huibregtse IL et al. In vitro and in vivo down-regulation of regulatory T cell activity with a peptide inhibitor of TGF-beta 1. J Immunol 2008; 181(1): 126–135.
15. Lieberman J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat Rev Immunol 2003; 3(5): 361–370.
16. Cao X. Regulatory T cells and immune tolerance to tumors. Immunol Res 2010; 46(1–3): 79–93.
17. Oderup C, Cederbom L, Makowska A et al. Cytotoxic T lymphocyte antigen-4 dependent down-modulation of costimulatory molecules on dendritic cells in CD4+CD25+ regulatory T-cell-mediated suppression. Immunology 2006; 118(2): 240–249.
18. Beyer M, Kochanek M, Giese T et al. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood 2006; 107(10): 3940–3949.
19. Feyler S, von Lilienfeld-Toal M, Jarmin S et al. CD4+CD25+FoxP3+ regulatory T cells are increased whilst CD3+CD4-CD8-αβ TCR+ Double Negative T cells are decreased in the peripheral blood of patients with multiple myeloma which correlates with disease burden. Br J Haematol 2009; 144(5): 686–695.
20. Prabhala RH, Neri P, Bae JE et al. Dysfunctional T regulatory cells in multiple myeloma. Blood 2006; 107(1): 301–304.
21. Gupta R, Ganeshan P, Hakim M et al. Significantly reduced regulatory T cell population in patients with untreated multiple myeloma. Leuk Res 2010. Epub of print.
22. Brimnes MK, Vangsted AJ, Knudsen LM et al. Increased level of both CD4+FOXP3+ regulatory T cells and CD14+HLA-DR–/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol 2010; 72(6): 540–547.
23. Atanackovic D, Cao Y, Luetkens T et al. CD4+CD25+FOXP3+ T regulatory cells reconstitute and accumulate in the bone marrow of patients with multiple myeloma following allogeneic stem cell transplantation. Haematologica 2008; 93(3): 423–430.
24. Wang X, Zheng J, Liu J et al. Increased population of CD4 (+) CD25 (high) regulatory T cells with their higher apoptotic and proliferating status in peripheral blood of acute myeloid leukemia patients. Eur J Haematol 2005; 75(6): 468–476.
25. Yang ZZ, Novak AJ, Stenson MJ et al. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T-cells in B-cell non-Hodgkin lymphoma. Blood 2006; 107(9): 3639–3646.
26. Giannopoulos K, Schmitt M, Własiuk P et al. The high frequency of T regulatory cells in patients with B-cell chronic lymphocytic leukemia is diminished through treatment with thalidomide. Leukemia 2008; 22(1): 222–224.
27. Quach H, Ritchie D, Stewart AK et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 2010; 24(1): 22–32.
Štítky
Detská onkológia Chirurgia všeobecná Onkológia
Článok vyšiel v časopiseKlinická onkologie
Najčítanejšie tento týždeň
2011 Číslo Supplementum 1- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejasný stín na plicích – kazuistika
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Fixní kombinace paracetamol/kodein nabízí synergické analgetické účinky
-
Všetky články tohto čísla
- Multiple Myeloma
- Monoclonal Gammopathy of Undeterminated Significance: Introduction and Current Clinical Issues
- Sample Processing and Methodological Pitfalls in Multiple Myeloma Research
- Flow Cytometry in Monoclonal Gammopathies
- Flow Cytometric Phenotyping and Analysis of T Regulatory Cells in Multiple Myeloma Patients
- Genomics in Multiple Myeloma Research
- Polymorphisms Contribution to the Determination of Significant Risk of Specific Toxicities in Multiple Myeloma
- Oligonucleotide-based Array CGH as a Diagnostic Tool in Multiple Myeloma Patients
- Radiotherapeutic methods
- Visualization of Numerical Centrosomal Abnormalities by Immunofluorescent Staining
- Impact of Nestin Analysis in Multiple Myeloma
- Editorial (EN)
- Editorial (CZ)
- List of authors and reviewers
- Klinická onkologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Multiple Myeloma
- Flow Cytometric Phenotyping and Analysis of T Regulatory Cells in Multiple Myeloma Patients
- Monoclonal Gammopathy of Undeterminated Significance: Introduction and Current Clinical Issues
- Flow Cytometry in Monoclonal Gammopathies
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



