-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Skin grafting on amputated lower limb, norepinephrine-induced ischemic limb necrosis – case report
Authors: K. Efthymiou; J. Kaťuchová; J. Radoňak; M. Kňazovický; J. Iľková; D. Tomašurová
Authors place of work: st Department of Surgery, Faculty of Medicine, Pavol Jozef Šafárik University in Košice and University Hospital Košice, Slovakia 1
Published in the journal: ACTA CHIRURGIAE PLASTICAE, 65, 3-4, 2023, pp. 150-154
doi: https://doi.org/10.48095/ccachp2023150Introduction
Full-thickness skin graft (FTSG) consists of complete epidermis and dermis. It is an option to repair defects of the lower leg [1]. FTSG is durable, exhibits minimal contraction, and usually gives good cosmetic effect [2,3].
Septic shock was defined by Singer et al. as circulatory, cellular, and metabolic abnormalities in septic patients. It is presenting as fluid-refractory hypotension due to peripheral vasodilation requiring vasopressor therapy with associated tissue hypoperfusion (lactate > 2 mmol/L) [4]. Peripheral vasodilation occurs after the failure of normal mechanisms to vasoconstrict vascular smooth muscle [5]. Sepsis is defined as life threatening organ dysfunction caused by a dysregulated host response to infection [6].
Septic shock in patients is characterized by vasoplegia and vascular endothelial injury that causes disseminated Intravascular coagulopathy (DIC) [7].
According to current guidelines, fluid replacement and vasopressor infusion must be titrated to increase mean arterial pressure (MAP) to 65 mmHg, including maintenance of central venous pressure (CVP) at 8–12 mmHg [8–10]. Hypotension due to relative and absolute hypovolemia is treated first with fluid administration [11]. Although due to sepsis-related systemic vasodilation, vasopressor therapy is fundamental in septic shock. It aims at correcting vascular tone depression and then at improving organ perfusion pressure [11]. Norepinephrine (NE) as a potent α-adrenergic agonist increases blood pressure primarily through its vasoconstrictive properties [12].
In the event of refractory septic shock, high dose of NE or combination of NE with vasopressin is used [13]. Vasopressin infusion decreases the dose requirement of NE, increases urine output and creatinine clearance, and sometimes decreases cardiac output [14,15]. Some studies defined, NE high dose by a cut--off value ranging from 0.5 µg/kg/min to 2 µg/kg/min [16]. However, Brown SM et al. defined a high dose of NE as more than 1 μg/kg/min [17]. According to the studies, 90% of patients receiving > 1 μg/kg/min of NE died [18]. A high dose of NE was associated with a high mortality [16] and ischemic complications [19–21].
Levy et al., in their systemic review and qualitative meta-analysis concluded that there is insufficient evidence to support that limb and digit ischemic necrosis is a potential complication of high-dose vasopressor therapy [22]. Herein we report a case of digit ischemic necrosis as a complication of high-dose vasopressor therapy in a patient with septic shock. An interesting fact is that the cause of infection was not identified. It can be described as a septic shock without documented infection an uncommon entity reported by Reyes et al. 1999 [23].
Fig. 1. Appearance of right hand (dorsal view). 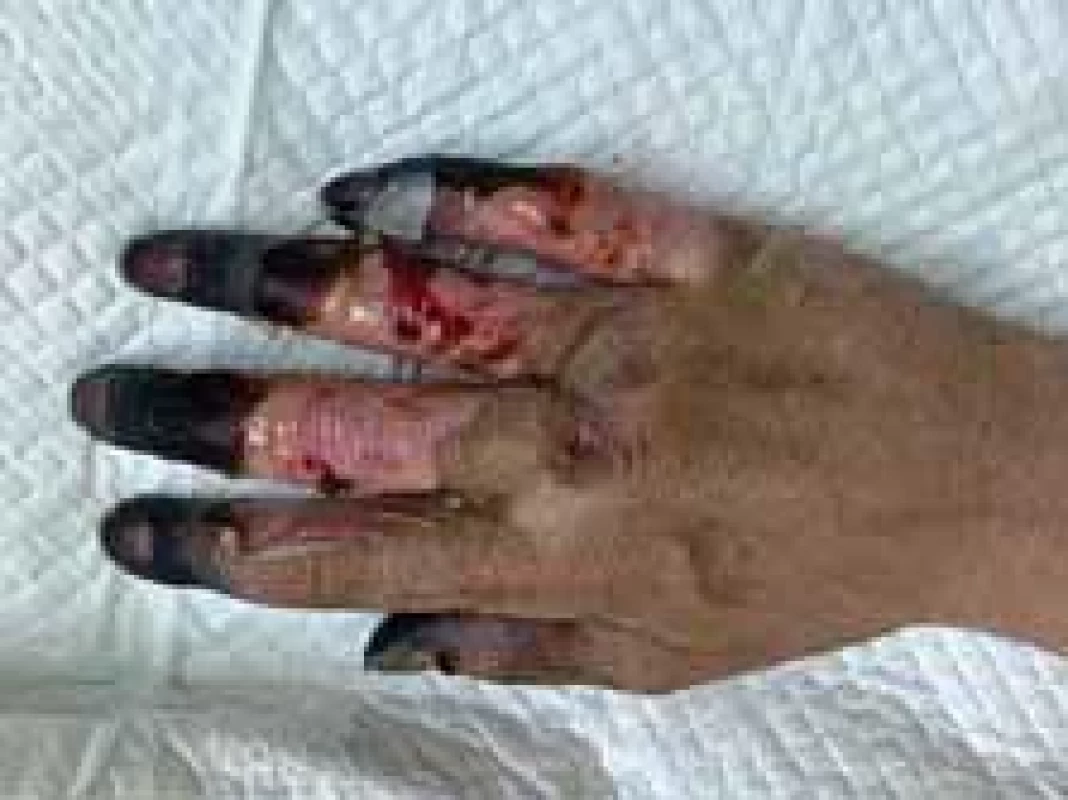
Fig. 2. Appearance of right hand (palm view). 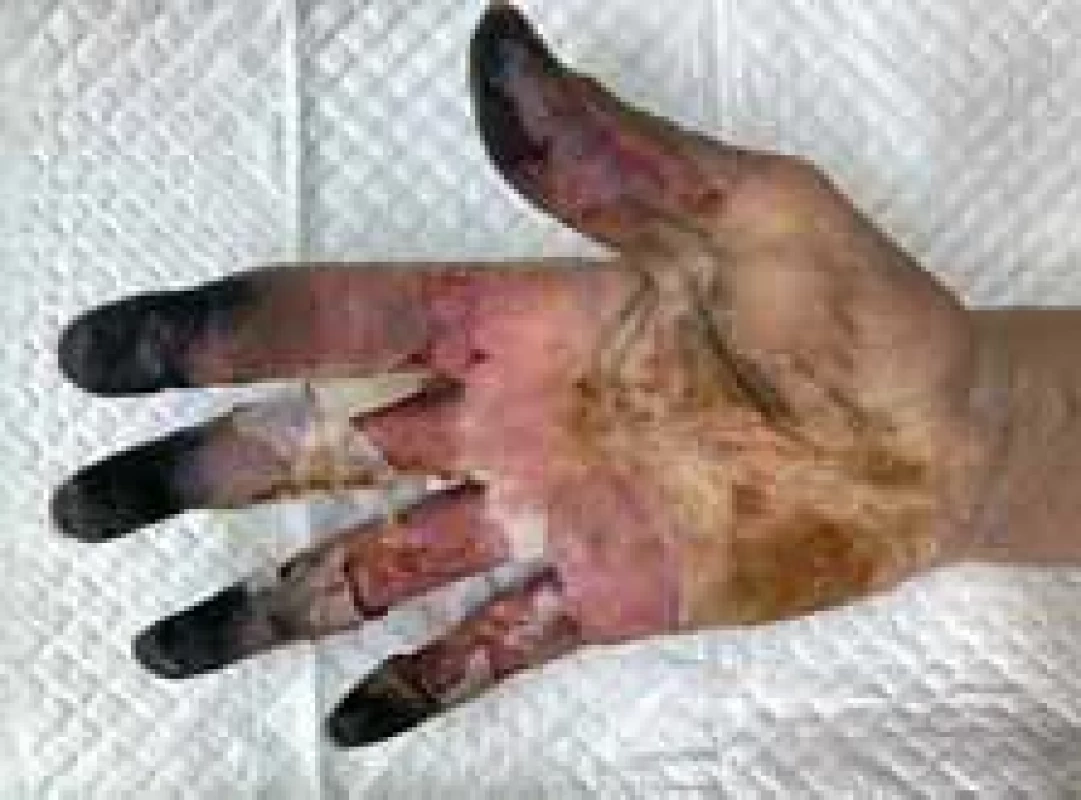
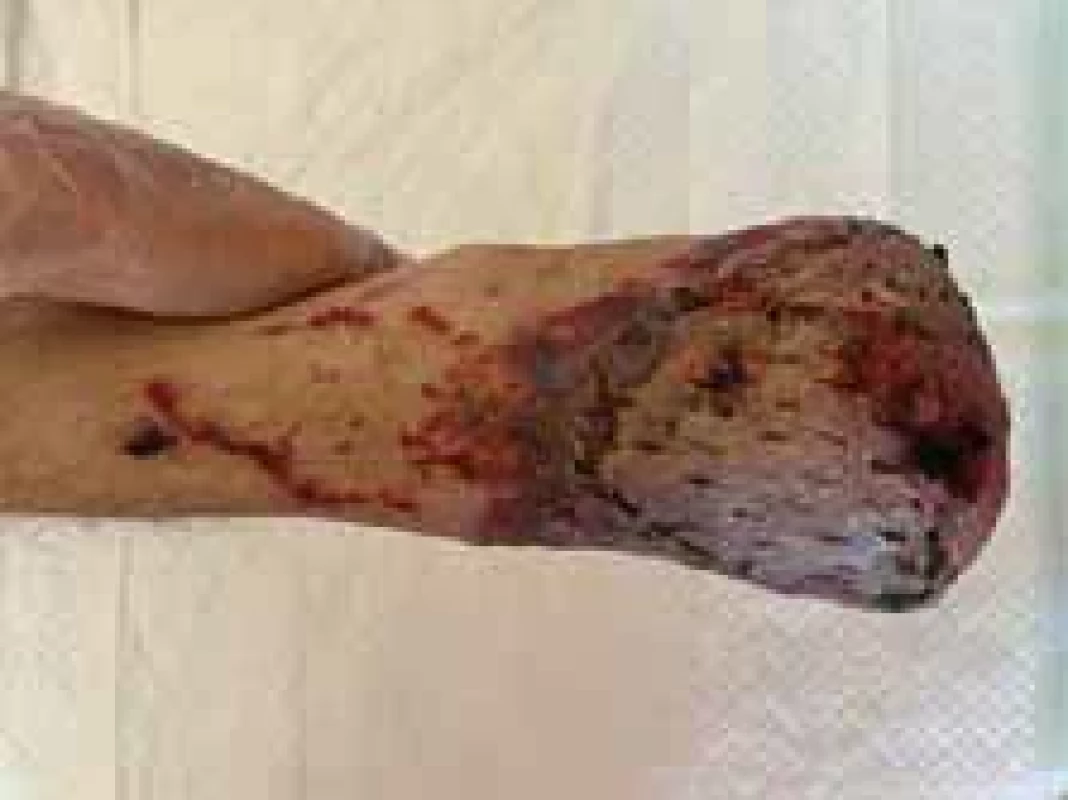
Fig. 3. Appearance of right foot (dorsal view). 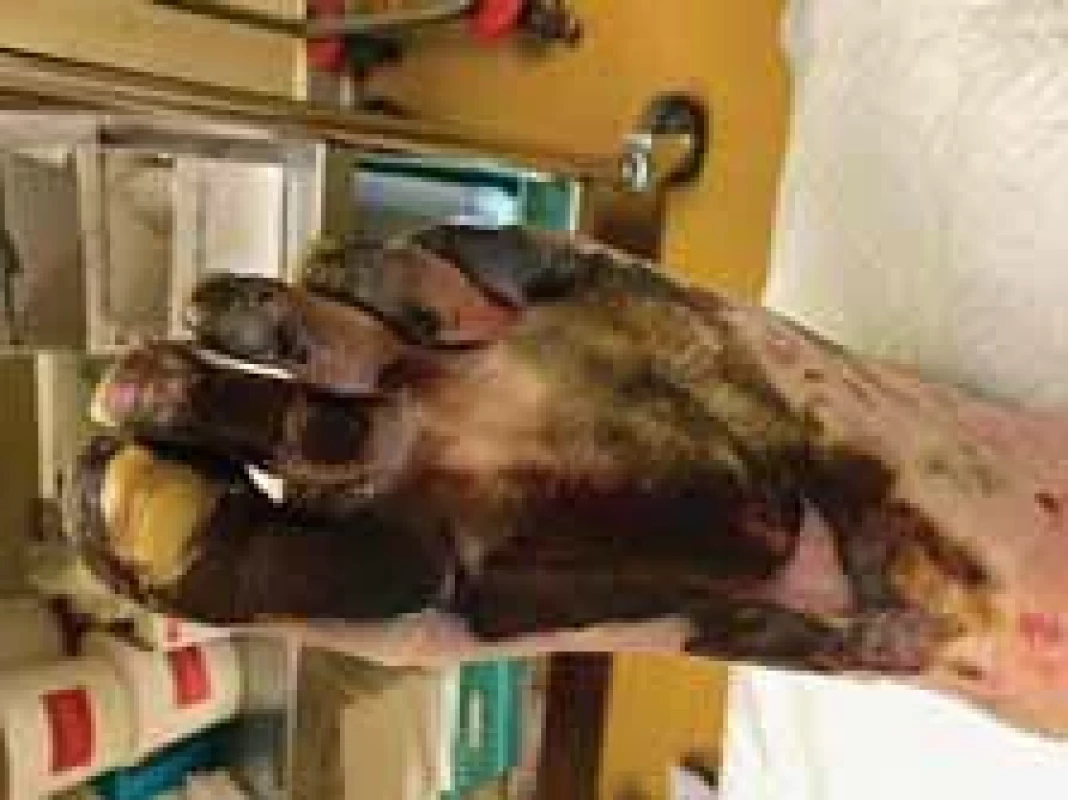
Fig. 4. Appearance of right foot with necrosis of toes and metatarsals (plantar view). 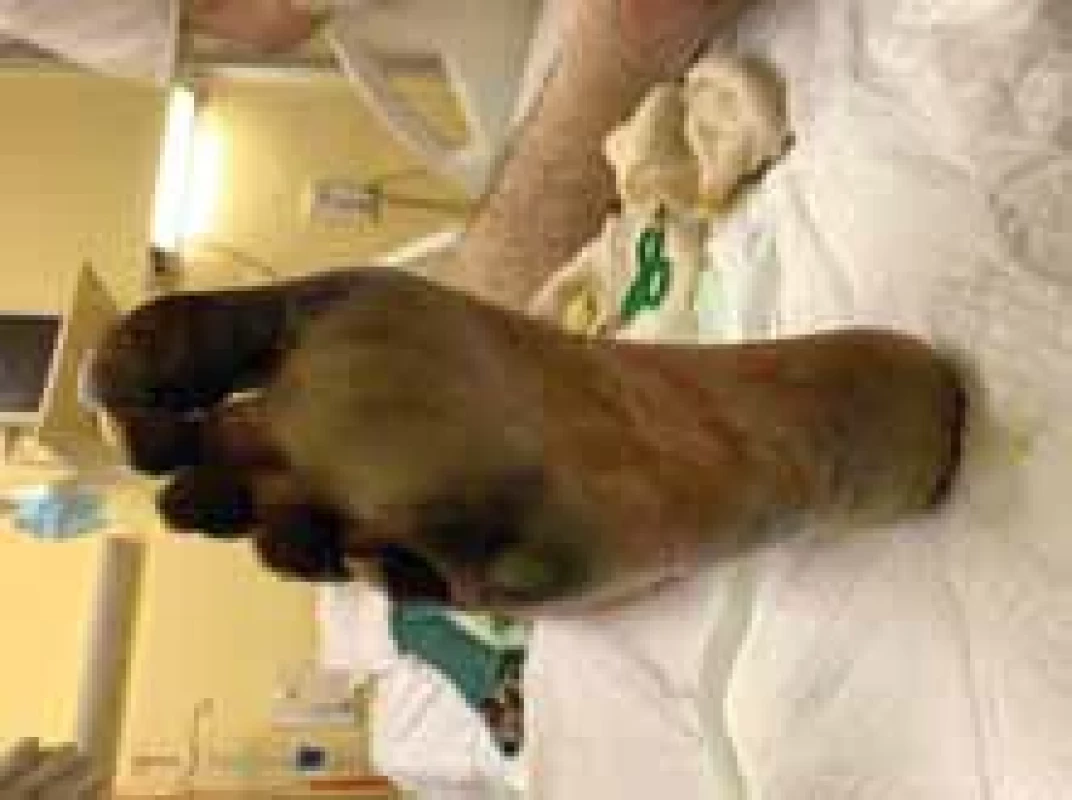
Fig. 5. Appearance of left foot (plantar view). 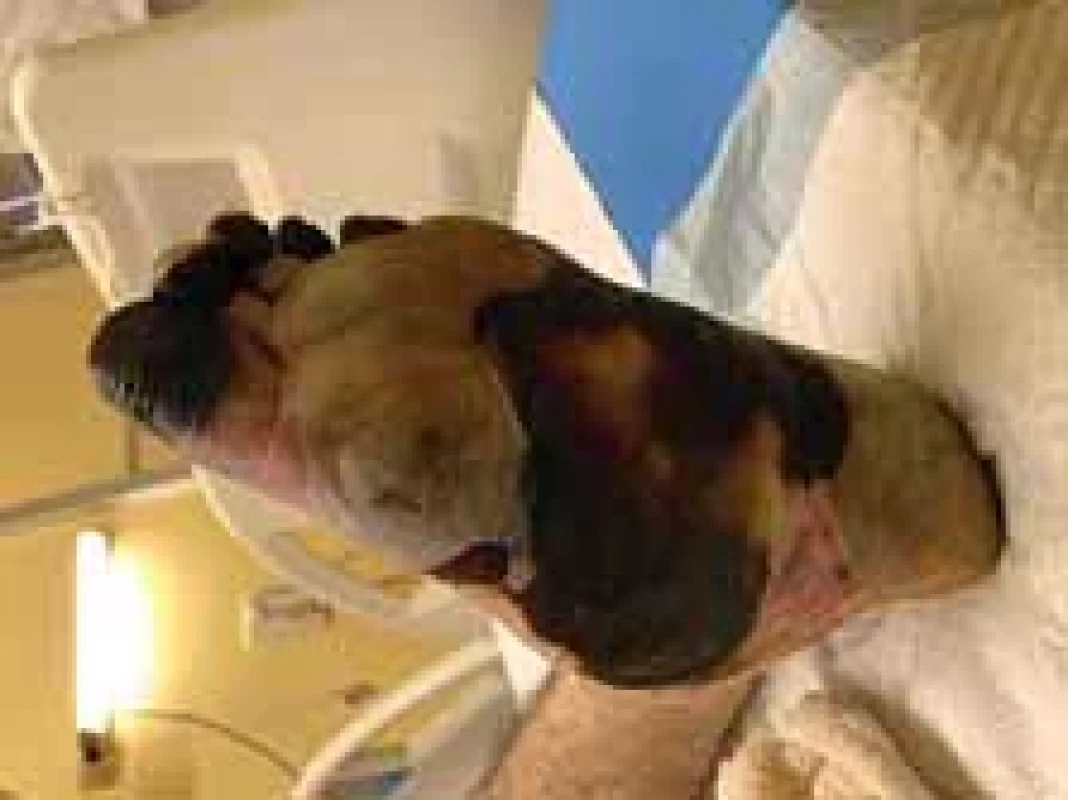
Description of the case
A 32-year-old man with a history of alcohol abuse, acute pancreatitis relapses, liver cirrhosis, and hypersplenism with associated hypocoagulation was admitted to our clinic for hematemesis. Gastroscopy confirmed the presence of gastroesophageal varices with haemorrhagic gastritis. The patient was treated with transfusions, vitamin K, and haemostatic drugs. The 3rd day of his hospitalization, his medical condition was complicated by a hypoglycemic coma. The patient was also hypotensive T: 80/50 mmHg with vasopressors, tachycardic P: 125/min, CVP: –5 mmHg, with febrility T: 40 °C. Laboratory results are demonstrated in the Tab. 1.
Tab. 1. Laboratory examination. 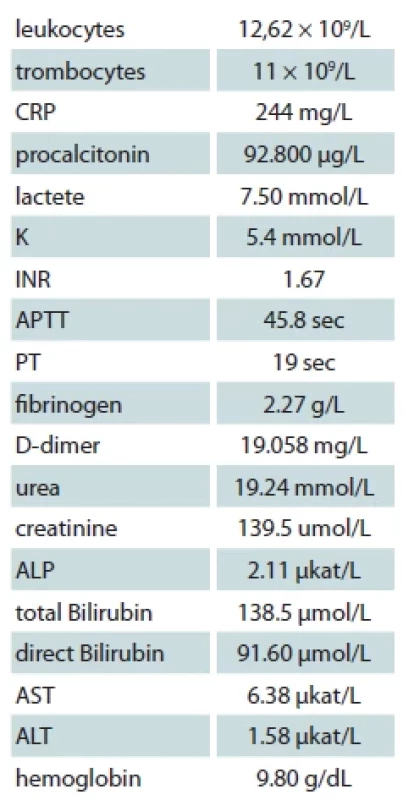
CRP – C-reactive protein, INR – international normalized ratio, APTT – activated parcial tromboplastine time, PT – prothrombin time, ALP – alka - line phosphatase, AST – aspartate transaminase, ALT – alanine aminotransferase Immediately was administered 40 mL of 40% glucose and later 500 mL of 10% glucose. The fluid resuscitation was done according to the recent International Guidelines for Management of Sepsis and Septic Shock 2021. The patient was hydrated with 30 mL/kg of IV isotonic crystalloid fluids within the first 3 hrs of resuscitation.
NE was given in a dose of 0,5 μg/kg/min. Because of persistent hypotension, the NE dose was increased to 1 μg/kg/min. The patient was moved to our hospital’s department of anaesthesiology and critical care for stabilization and further management of septic shock. The patient was intubated and placed on invasive ventilation. For the following 2 days, NE administration was continued at lower doses of 0.5 mcg/kg/min with the combination of vasopressin in 4 mL/hour. Approximately 5 L of fluids were administered to the patient daily.
Lactic acidosis was treated with bicarbonate. Hypocoagulation and thrombocytopenia were corrected with fresh frozen plasma and concentrated thrombocytes, respectively. X-ray of the lungs was taken, and pneumonia was excluded. Blood culture was taken for aerobic and anaerobic bacteria and fungi from the central venous catheter; culture from the endotracheal tube, rectum, and urine have all shown negative results. Other clinical conditions associated with severe inflammatory response, such as pancreatitis and trauma, were excluded. Empirical administration of broad-spectrum antibiotics continued as the source or pathogen of infection that led to sepsis was not found.
On the 4th day of administration and 24 hrs after vasopressor administration, symmetrical necrosis of the digits of the upper and lower extremities was identified. The appearance of the extremities is seen in the following pictures (Fig. 1–5). The patient was referred to an orthopaedic surgeon who excluded bone fractures by seeing X-rays of the upper and lower limbs. Moreover, the vascular surgeon considered impossible to restore vascular perfusion to the vasculature as upper and lower limb doppler ultrasounds’ performance excluded thrombosis and atherosclerosis in affected vessels.
Amputations of distal phalanges in the hands and amputation of metatarsal in the feet were performed on the 6th day of admission and the 3rd day after necrosis was identified. The following pictures demonstrate the appearance of the limbs after the operation (Fig. 6–12). It was concluded that the cause of necrosis was microvascular spasms due to vasopressors. That is why amputation of necrotic parts was indicated. Vacuum assisted closure therapy was used on both legs after amputation to speed up wound healing, to improve tissue perfusion, to suction exudates and to facilitate removal of bacteria from the wounds in the lower limbs (Fig. 13).
One month after amputation was the patient referred to a plastic surgeon to cover the remaining acral parts with a skin graft. The large defects were covered with full-thickness skin autografts from the thighs (Fig. 14). The patient was discharged from our department after 3 months of hospitalization. The appearance of the lower limb after FTSG transplantation a day before discharge is demonstrated in Fig. 15.
Fig. 6. Appearance of right hand after operation (dorsal view). 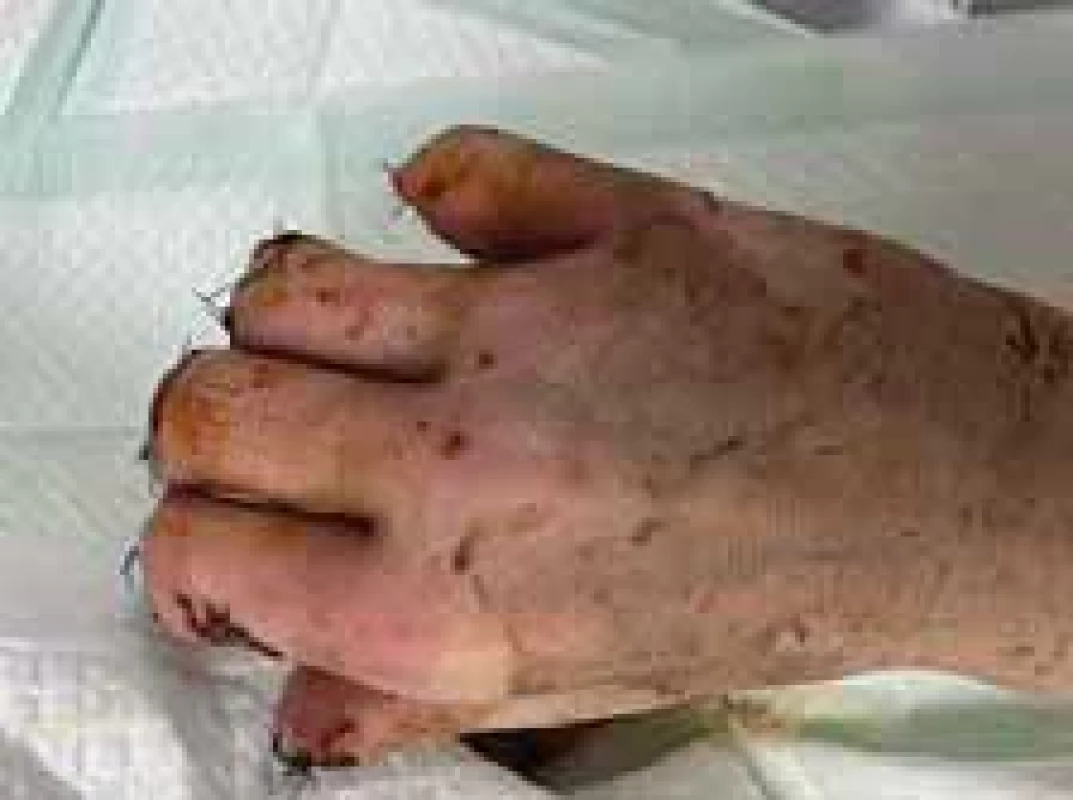
Fig. 7. Appearance of right hand after operation (frontal view). 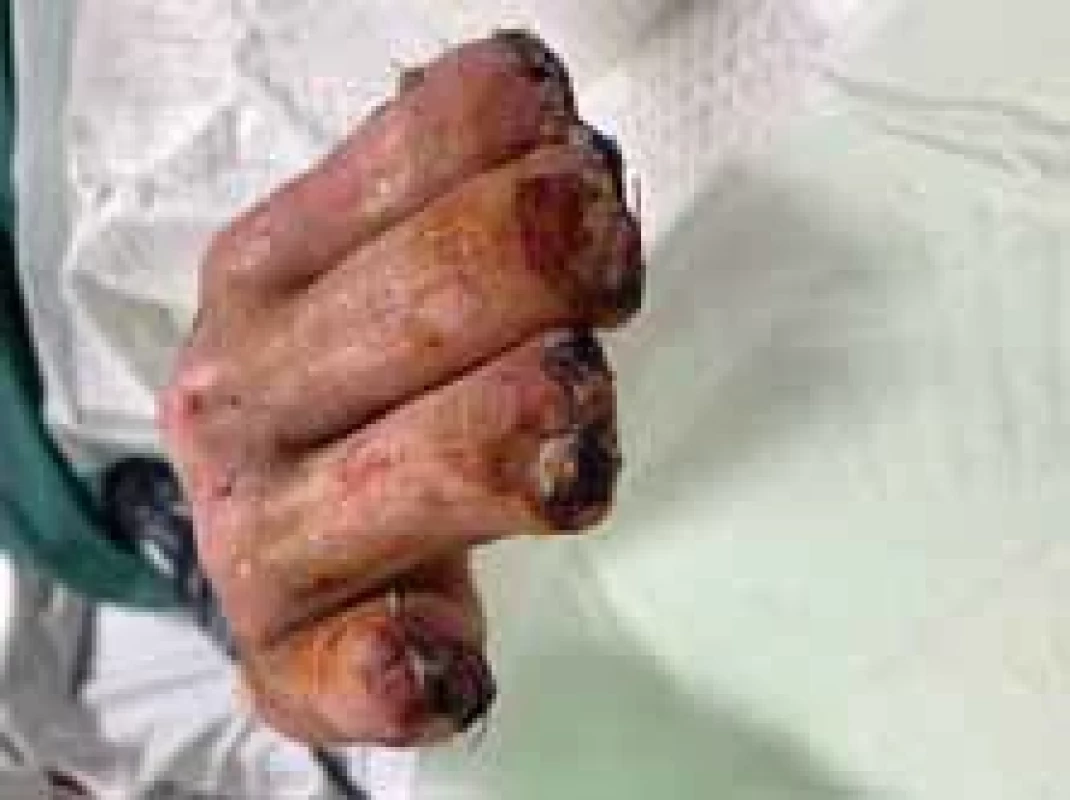
Fig. 8. Appearance of left hand after operation (frontal view). 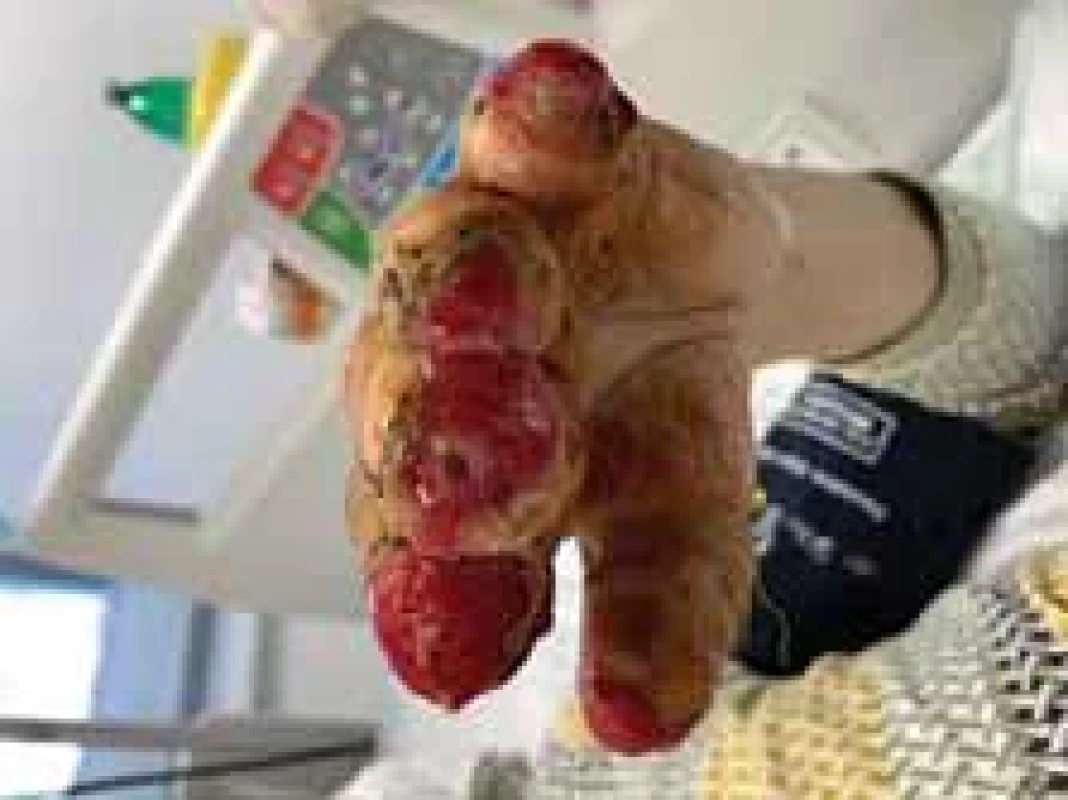
Fig. 9. Appearance of left hand after operation (palm view). 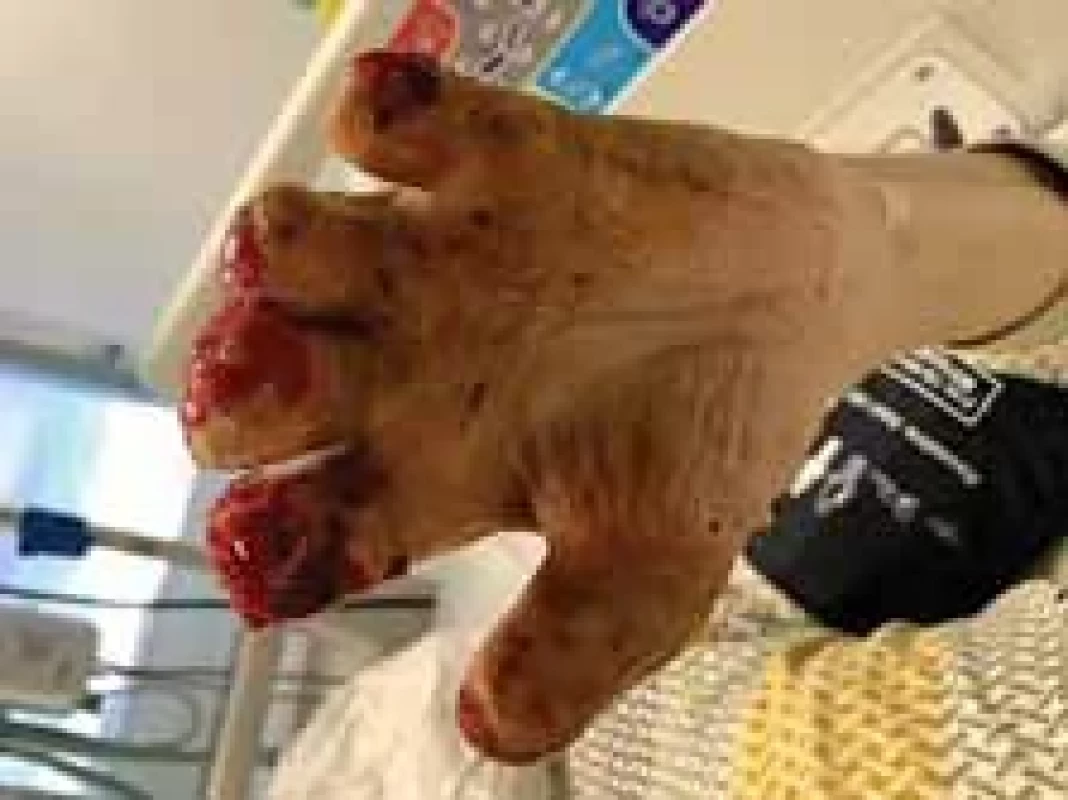
Fig. 10. The appearance of hands. A day before discharge (frontal view). 
Fig. 11. The appearance of right foot after amputation and removal of debridement (lateral view). 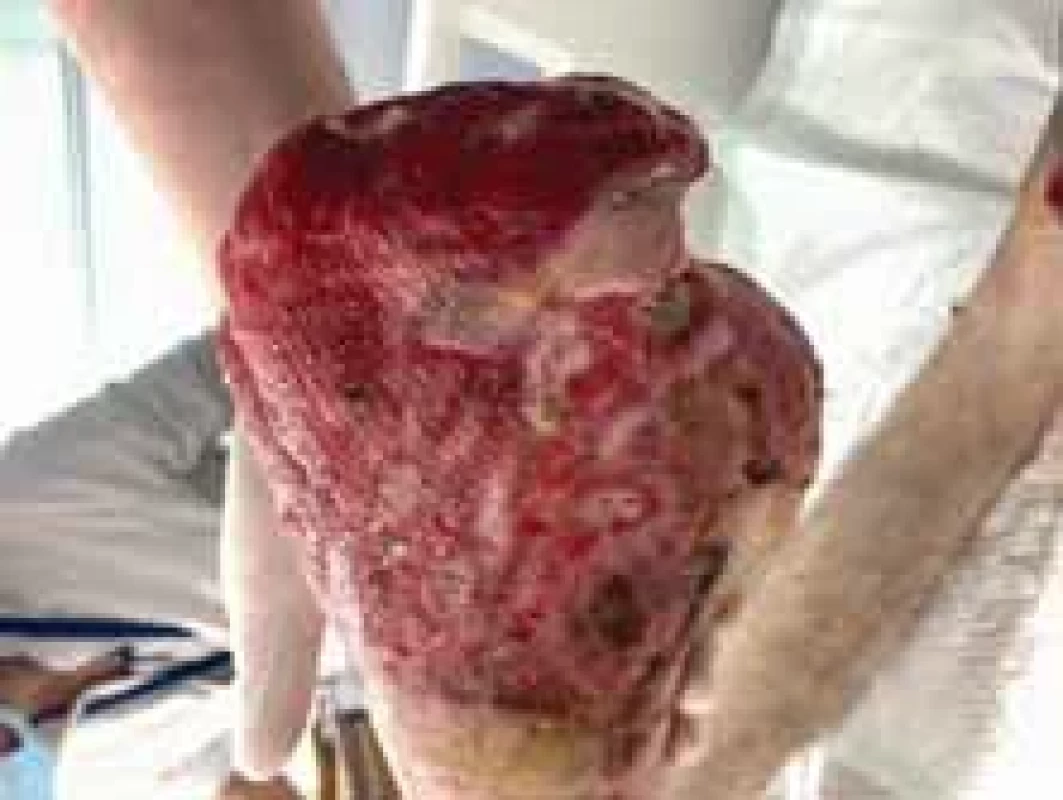
Fig. 12. The appearance of left foot after amputation and removal of debridement (plantar view). 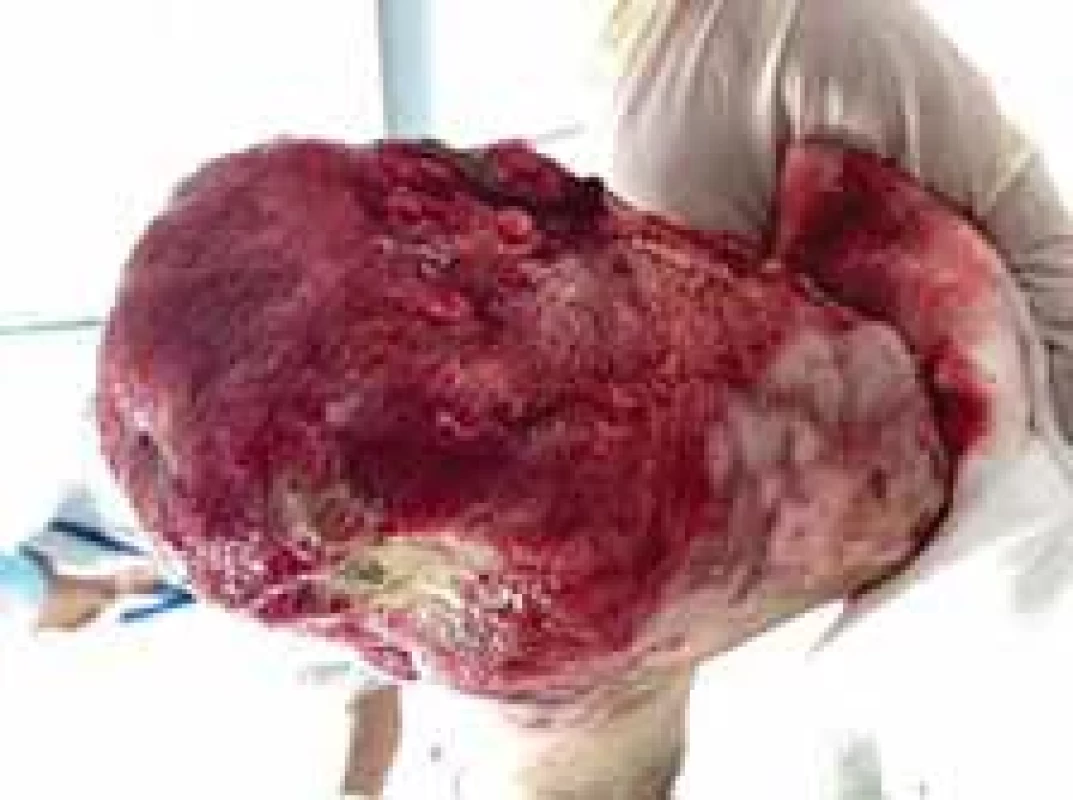
Fig. 13. Application of vacuum assisted closure (VAC) therapy after open transmetatarsal amputation. 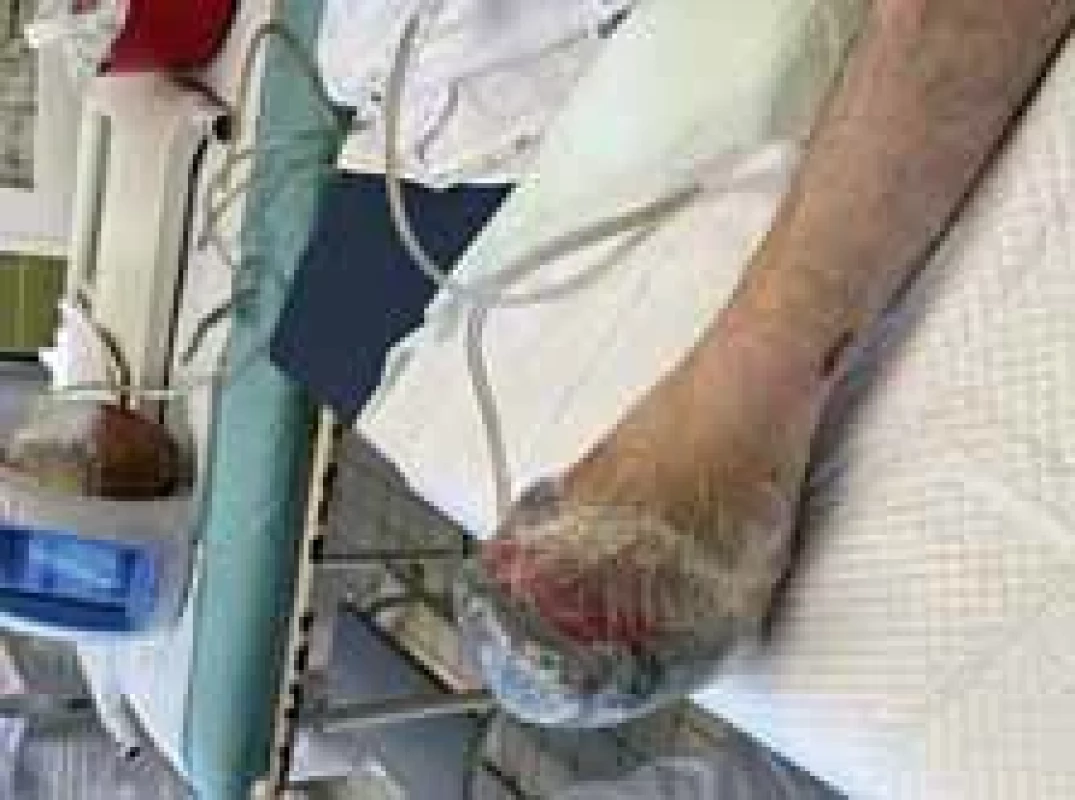
Fig. 14. The appearance of the lower limb during operation. Full-thickness skin grafts are visible. 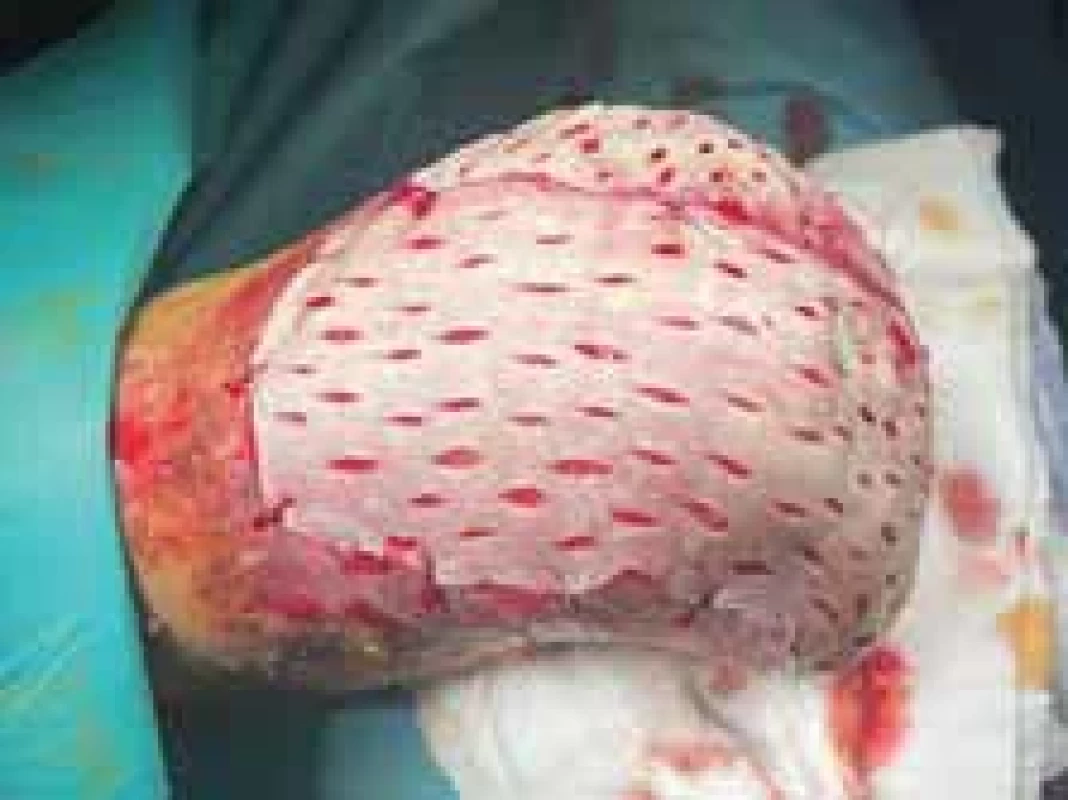
Discussion
FTSG are durable and can provide skin coverage in wounds in which healing by second intention, primary closure, or flap repair would not be optimal. Durability is important on the lower limb, which is subject to inevitable sheer forces associated with gait [2]. Moreover, FTSG offers reduced donor site morbidity because primary closure leads to quicker healing and less pain [3]. In our case, FTSG was the best available choice.
The third international consensus conference in 2016 defined Sepsis as life-threatening organ dysfunction caused by a dysregulated host response to infection. Septic shock is defined as a subset of sepsis in which circulatory, cellular, or metabolic abnormalities are associated with increased mortality risk. Clinical parameters to identify patients with septic shock consist of vasopressor requirement to maintain MAP of 65 mmHg or greater and a serum lactate level greater than 2 mmol/L in the absence of hypovolemia [4,6]. In this case, clinical parameters fit the definition of septic shock, but the source of infection was not pointed out, either from clinical or microbiological evidence. It is potentially a rare case of septic shock without documented infection [23].
Patients with septic shock require aggressive fluid resuscitation and vasopressor therapy [24]. The first-line vasopressor in septic shock is NE. Vasopressin and its analogues are second-line vasopressors that can also be combined with NE in cases of refractory hypotension instead of increasing NE doses [25]. NE increases blood pressure primarily through its vasoconstrictive properties with little effect on the heart rate [12]. In shock, vasopressin acts as a potent vasoconstrictor via V1a receptors on vascular smooth muscle [24]. High doses of NE are associated with high mortality and complications as mesenteric ischemia and peripheral limb necrosis [20,22]. Liver dysfunction with decreased release of regulatory anticoagulants such as anti-thrombin 3 and protein C appears to increase the risk of symmetrical peripheral necrosis [26]. Symmetrical peripheral gangrene is a devastating complication of septic shock due to DIC driven micro-thrombosis [27]. In our case, necrosis was caused by vasopressor induced vasoconstriction due to high dose NE, as DIC was excluded according to the Modified diagnostic criteria for DIC from the Japanese Society on Thrombosis and Haemostasis [28]. Remarkable ischemic necrosis of the limbs developed from microvascular spasms caused by the high dose of NE.
Conclusion
Septic shock with non-documented infection is a rare case, which is why it is important to conduct an early and aggressive search for septic origin in all septic patients. NE use in high doses for severe septic shock can cause severe complications such as ischemic limb necrosis. It is essential to consider its use before administration. However, a combination of lower NE doses with vasopressin may help to avoid such unfortunate results. Amputation of necrotic parts is the only available treatment. FTSG transplantation, rehabilitation and prosthesis have a good impact on the outcomes.
Roles of authors
Kleanthia Efthymiou, MD – author made a substantial contribution to the conception, design of the work and the handling of data contained within the manuscript;Prof. Jana Kaťuchová, MD, PhD, MBA – co-author made a substantial contribution to the conception;Prof. Jozef Radoňak, MD, Csc, MPH – co-author made a significant contribution to the conception;Miloš Knazovický, MD, PhD, MPH – co-author made a significant contribution for handling of data contained within the manuscript;Jana Iľková, MD – co-author made a significant contribution for handling of data contained within the manuscript;Diana Tomašurová, MD – co-author made a significant contribution to the conception and design of the work.Disclosure
The authors have no conflicts of interest to disclose. The authors declare that this study has received no financial support. All procedures per formed in this study involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the Helsinki declaration and its later amendments or comparable ethical standards.
Zdroje
1. Rao K., Tillo O., Dalal M. Full thickness skin graft cover for lower limb defects following excision of cutaneous lesions. Dermatol Online J. 2008, 14 (2): 4.
2. Oganesyan G., Jarell AD., Srivastava M., et al. Efficacy and complication rates of full‐thickness skin graft repair of lower extremity wounds after Mohs micrographic surgery. Dermatol Surg. 2013, 39 (9): 1334–1339.
3. Audrain H., Bray A., De Berker D. Full-thickness skin grafts for lower leg defects: an effective repair option. Dermatol Surg. 2015, 41 (4): 493–498.
4. Singer M., Deutschman CS., Seymour CW., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016, 315 (8): 801–810.
5. Russell JA., Rush B., Boyd J. Pathophysiology of septic shock. Crit Care Clin. 2018, 34 (1): 43–61.
6. Gotts JE., Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016, 353: i1585.
7. Iba T., Levy JH. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J Thromb Haemost. 2018, 16 (2): 231–241.
8. Dellinger RP., Levy MM., Rhodes A., et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013, 39 (2): 165–228.
9. Asfar P., Meziani F., Hamel JF., et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014, 370 (17): 1583–1593.
10. Lin C., Chen T., Lu P., et al. Lower initial central venous pressure in septic patients from long-term care facilities than in those from the community. J Microbiol Immunol Infect. 2014, 47 (5): 422–428.
11. Rhodes A., Evans L., Alhzzani W., et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock. Intensive Care Med. 2017, 43 (3): 304–377.
12. Scheeren TW., Bakker J., De Backer D., et al. Current use of vasopressors in septic shock. Ann Intensive Care. 2019, 9 (1): 20.
13. Auchet T., Regnier MA., Girerd N., et al. Out-come of patients with septic shock and high-dose vasopressor therapy. Ann Intensive Care. 2017, 7 (1): 43.
14. Landry DW., Levin HR., Gallant EM., et al. Vasopressin pressor hypersensitivity in vasodilatory septic shock. Crit Care Med. 1997, 25 (8): 1279–1282.
15. Malay MB., Ashton Jr RC., Landry DW., et al. Low-dose vasopressin in the treatment of vasodilatory septic shock. J Trauma. 1999, 47 (4): 699–703.
16. Bassi E., Park M., Azevedo LCP. Therapeutic strategies for high-dose vasopressor-dependent shock. Crit Care Res Pract. 2013, 2013 : 654708.
17. Brown SM., Lanspa MJ., Jones JP., et al. Survival after shock requiring high-dose vasopressor therapy. Chest. 2013, 143 (3): 664–671.
18. Martin C., Medam S., Antonini F., et al. Norepinephrine: not too much, too long. Shock. 2015, 44 (4): 305–309.
19. Auchet T., Regnier MA., Girerd N., et al. Out-come of patients with septic shock and high-dose vasopressor therapy. Ann Intensive Care. 2017, 7 (1): 43.
20. Hamzaoui O., Scheeren TW., Teboul JL. Norepinephrine in septic shock: when and how much? Curr Opin Crit Care. 2017, 23 (4): 342–347.
21. Warkentin TE. Microvascular thrombosis and ischaemic limb losses in critically ill patients. Hämostaseologie. 2019, 39 (1): 6–19.
22. Levy JH., Ghadimi K., Faraoni D., et al. Ischemic limb necrosis in septic shock: what is the role of high‐dose vasopressor therapy? J Thromb Haemost. 2019, 17 (11): 1973–1978.
23. Reyes WJ., Brimioulle S., Vincent JL. Septic shock without documented infection: an uncommon entity with a high mortality. Intensive Care Med. 1999, 25 (11): 1267–1270.
24. Shi R., Hamzaoui O., De Vita N., et al. Vasopressors in septic shock: which, when, and how much? Ann Transl Med. 2020, 8 (12): 794.
25. O’Callaghan DJ., Gordon AC. What’s new in vasopressin? Intensive Care Med. 2015, 41 (12): 2177–2179.
26. Warkentin TE., Pai M. Shock, acute disseminated intravascular coagulation, and microvascular thrombosis: is “shock liver” the unrecognized provocateur of ischemic limb necrosis? J Thromb Haemost. 2016, 14 (2): 231–235.
27. Warkentin TE. Ischemic limb gangrene with pulses. N Engl J Med. 2015, 373 (7): 642–655.
28. Wada H., Takahashi H., Uchiyama T., et al. The approval of revised diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J. 2017, 15 : 17.
Kleanthia Efthymiou, MD
Trieda SNP 1
040 11 Košice
Slovakia
e-mail: kleanthiaefthimiou@hotmail.comSubmitted: 1. 6. 2023
Accepted: 4. 1. 2024Štítky
Chirurgia plastická Ortopédia Popáleninová medicína Traumatológia
Článok vyšiel v časopiseActa chirurgiae plasticae
Najčítanejšie tento týždeň
2023 Číslo 3-4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Srovnání analgetické účinnosti metamizolu s ibuprofenem po extrakci třetí stoličky
- Fixní kombinace paracetamol/kodein nabízí synergické analgetické účinky
-
Všetky články tohto čísla
- Editorial
- Diagnosis and treatment of Eagle’s syndrome and possible complications
- Scalp arteriovenous malformations – 20 years of experience in a tertiary healthcare centre
- The comparison of effectivity in breast cancer prevention between skin sparing and subcutaneous mastectomy – 20 years of experience
- Avascular necrosis of the maxilla after orthognathic surgery, a devastating complication? A systematic review of reported cases and clinical considerations
- 3D maxillofacial surgery planning – one decade development of technology
- A primary cutaneous carcinosarcoma of the retro auricular region, how to treat and literature review
- Combination of cable ties and barbed sutures for fasciotomy closure – two case reports
- Skin grafting on amputated lower limb, norepinephrine-induced ischemic limb necrosis – case report
- Abdominal wall reconstruction for extensive necrosis following abdominoplasty in a patient with subcostal scars – case report
- Acta chirurgiae plasticae
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Diagnosis and treatment of Eagle’s syndrome and possible complications
- Avascular necrosis of the maxilla after orthognathic surgery, a devastating complication? A systematic review of reported cases and clinical considerations
- 3D maxillofacial surgery planning – one decade development of technology
- Skin grafting on amputated lower limb, norepinephrine-induced ischemic limb necrosis – case report
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy