-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Advanced heart failure and cardiac arrhythmia in a young adult survivor of childhood cancer
Závažné zlyhávanie srdca a arytmia po protinádorovej liečbe podávanej v detstve
Cieľom kazuistiky je popísať prípad mladého pacienta, v detstve úspešne liečeného pre non-Hodgkinov lymfóm chemoterapiou s obsahom anthracyklínu doxorubicínu s neskorším rozvojom príznakov závažného kardiovaskulárneho poškodenia 27 rokov od diagnostiky lymfómu. Pacient je dlhodobo v kompletnej remisii onkologického ochorenia. U pacienta bola započatá liečba chronického srdcového zlyhávania v súlade s najnovšími odporúčaniami vrátane implantácie kardioverter-defibrilátora, ako primárnej prevencie náhlej kardiálnej smrti. Momentálne je pacient čakateľom na transplantáciu srdca.
Klíčová slova:
srdcové zlyhávanie – antracyklíny – implantovatelný kardioverter-defibrilátor – arytmia – neskorá kardiotoxicita
Authors: Xénia Faktorová 1; Milan Luknár 2,3; Zuzana Zelinková 1; Lucia Horniaková 4; Eva Mikušková 5; Michal Šašov 6; Mária Szántová 4; Viliam Mojto 4; Beata Mladosievičová 7
Authors place of work: Department of Internal Medicine of the Slovak Medical University, University Hospital – St. Michael’s Hospital, a. s, Bratislava 1; Department of Heart Failure and Heart Transplantation National Institute of Cardiovascular Diseases, Bratislava 2; Department of Cardiology, Faculty of Medicine, Comenius University and National Institute of Cardiovascular Diseases, Bratislava 3; Department of Oncohematology Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava 5; Department of Arrhytmias and Cardiac Stimulation National Institute of Cardiovascular Diseases, Bratislava 6; Institute of Pathological Physiology, Department of Clinical Pathophysiology, Faculty of Medicine, Comenius University, Bratislava 7; rd Department of Internal Medicine, Faculty of Medicine, Comenius University and University Hospital, Bratislava 43
Published in the journal: Vnitř Lék 2022; 68(E-1): 22-26
Category: Kazuistiky
Summary
The goal of this case report is to describe the young childhood cancer survivor who was treated for non‑Hodgkin lymphoma with chemotherapy containing anthracycline doxorubicin and who developed symptoms of serious cardiovascular damage 27 years after diagnosis of cancer. The patient is in long‑term complete remission of the lymphoma. He started guideline medical therapies for chronic heart failure and had a cardioverter defibrillator implanted for primary prevention of sudden cardiac death. He is currently a candidate for heart transplantation.
Keywords:
Arrhythmia – heart failure – anthracyclines – implantable cardioverter defibrillator – late cardiotoxicity
Introduction
Every year, 15 million people worldwide are diagnosed with cancer, of which about 300.000 are children. In developed countries, more than 2/3 of paediatric cancer patients have been cured in the last two decades and for some diagnoses (e.g., haematology‑oncological malignancies) the number of patients treated is even higher. However, 60% of paediatric cancer survivors experience serious or less serious long‑term and late adverse health outcomes after the cancer treatment. Serious late outcomes after a multiyear latent period also include cardiovascular (CV) complications (1). In cardiology practice, childhood cancer survivors who received cardiotoxic treatment require special long‑term attention and follow‑ups (2-5). Although there is a growing base of knowledge about cardiotoxicity with classical cytostatics, targeted (biological) hormonal treatment and tumour immunotherapy, anthracycline cardiotoxicity is the most studied and understood – acute, chronic (within 1 year after treatment) and late (after 1 year or even several decades after treatment) (6-8). Despite the irreversible anthracycline‑induced loss of cardiomyocytes, the heart is able to compensate this damage with no clinical manifestations for many years. As the risk factors accumulate and stressful situations increase (e.g., viral infections, excessive physical activity, increased somatic growth, pregnancy, surgeries), compensatory mechanisms cease to be effective and structural myocardial damage progresses to systolic dysfunction and clinical heart failure (1, 5, 9, 10).
Case analysis
We report a case of a 31-year‑old male patient of T ‑ cell non‑Hodgkin lymphoma. He was diagnosed at the age of 4 years in 1991. He underwent diagnostic thoracotomies followed by chemotherapy (doxorubicin (cumulative dose of 230 mg/m2), cyclophosphamide, vincristine, L‑asparaginase, methotrexate, mercaptopurine, cytarabine, teniposide, bleomycin (total dose of 22.5 mg/m2), prednisone)) of T‑cell non‑Hodgkin lymphoma. He achieved long‑term remission and has been monitored by his oncologist. He was also diagnosed with genetically confirmed, asymptomatic CADASIL syndrome (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) and hypothyroidism without the need for substitution therapy. In April 2019, he presented with a two months of non‑productive cough, night sweats, decreased appetite, fatigue and dyspnea on exertion (after a walking a few meters). He had no chest pain and no peripheral edema. The initial physical exam demonstrated clear lungs and no evidence of edema. The laboratory evaluation showed elevated NT‑proBNP (1423 ng/l). Serological examination (Chlamydia pneumoniae, Mycoplasma pneumoniae) was negative. Microbiological examination (nasal swabs/tonsils/urine/sputum) revealed no infectious agent. Chest X‑ray demonstrated pulmonary venous congestion. Transthoracic echocardiogram was obtained and revealed severely decreased left ventricular systolic function. The estimate left ventricular ejection fraction was 10%. There was global left ventricular hypokinesis. Right ventricular function was normal. The estimated right ventricular systolic pressure was elevated (50 mmHg) consistent with moderate pulmonary hypertension. (Tab. 1). PET‑CT scan did not show any evidence of lymphoma recurrence. Cardiomegaly and minimal fluidopericardium were seen. We obtained these results of previous echocardiographic examinations for comparison:
(2010): left ventricle slightly dilated, with preserved systolic and diastolic function, with diffuse hypokinesia, left ventricular ejection fraction 50-55%. Valves with good kinetics, sufficient.
(2016): neither dilatation nor left ventricular hypertrophy, which is without significant kinetics or global systolic dysfunction, was confirmed. No hemodynamically significant valve or short ‑ circuit disorder, left ventricular ejection fraction (LVEF) of 54%.
Tab. 1. Selected echocardiographic indicators 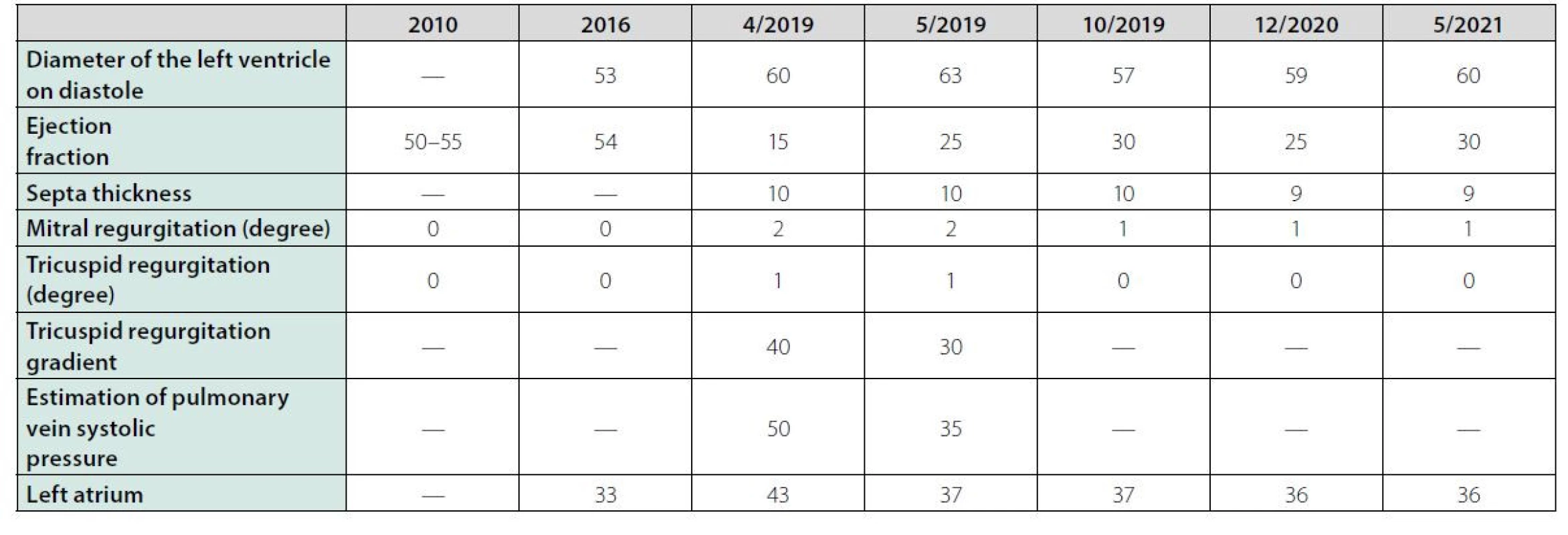
Based on the case history and results of the examinations, we diagnosed the condition as dilated cardiomyopathy, most likely on the basis of late anthracycline toxicity. In April 2019, at the beginning of hospitalization, we administered the beta‑blocker bisoprolol for sinus tachycardia and, after the first echocardiographic examination, we also indicated the ACE inhibitor perindopril, the diuretic furosemide and the selective aldosterone blocker eplerenone. Due to symptomatic manifestations of hypotension (dizziness, collapse conditions) and worsening of renal parameters, we had to discontinue both the beta‑blocker and the ACE inhibitor, titrate the dose of diuretics and indicate the selective sinoatrial node inhibitor ivabradine. The patient’s condition improved during the above treatment and the patient was discharged to outpatient care. The patient then adhered to a regime of restricted of physical activity and stress and after consultation with experts of the National Institute for Cardiovascular Diseases, he was monitored by a cardiologist. The patient was taking atorvastatin (for both higher total and LDL cholesterol), eplerenone, furosemide, trimetazidine, ivabradine and 1 month after being discharged (May 2019) he underwent echocardiography, that again, showed severe diffuse hypokinesis with the above valve defects, but with an increase in left ventricular ejection fraction from 10% to 25%. In August 2019, another echocardiography showed the same LVEF of 25% and concluded: dilated cardiomyopathy with left ventricular failure. Cardiac symptoms (dyspnoea) occurred mainly under high stress levels. In early October 2019, CT coronary angiography was performed where no atherosclerotic changes in the coronary arteries were identified. The lung parenchyma was without focal and infiltrative changes and without pleural effusion. In late October, ECG Holter monitor showed frequent monomorphic premature ventricular contractions (overall burden 10%) with right bundle branch block morphology. Sinus tachycardia and numerous ventricular extrasystoles were present at the time of subjective difficulties reported by the patient. No supraventricular extrasystole or atrial fibrillation was identified. As part of the primary prevention of sudden cardiac death, a single‑chamber cardioverter defibrillator (ICD Visia) was implanted in the patient in November 2019 at the Division of Arrhythmias and Cardiac Pacing of the National Institute for Cardiovascular Diseases in Bratislava. After increasing the dose of beta‑blocker, the incidence of ventricular extrasystole were reduced. Mild cardiomegaly, increased bronchovascular pattern, and elevated left diaphragm persisted on the follow‑up chest X‑ray (Figure 1). The follow‑up spirometry test documented a moderate restriction disorder with a moderate reduction in diffusion capacity. In October 2019, the NT‑proBNP level was 255 ng/l and the LVEF was 30%. The echocardiographic image is shown in Figure 2. During another follow‑up, in December 2020, the level of NT‑proBNP was 204 ng/l and LVEF was 25%. At another follow‑ap, in May 2021, the level of NT‑proBNP was 180 ng/l and LVEF was 30%. Subjectively, the patient feels good, has no chest pain, tolerates normal physical exertion without difficulty. The patient is treated by a cardiologist with the recommendation of regular blood pressure checks and adequate physical activity and the recommended treatment: beta‑blocker, ACE inhibitor, eplerenone, vitamin D and furosemide. The patient is a potential candidate for a heart transplant, which currently is not indicated for good functioning. Possible barriers in the future include the carrying of the gene for CADASIL syndrome.
Fig. 1. Chest X-ray of October 2019 
Fig. 2. Echocardiography image of October 2019 
Discussion
The present case report describes a patient, who at the age of four years was treated for T‑cell non‑Hodgkin lymphoma with chemotherapy containing doxorubicin at a cumulative dose of 230 mg/ m2. Even though this anthracycline dose is not high (the maximum cumulative dose in children is 400 mg/ m2), it cannot be considered safe, especially given the patient’s young age and stressful situations (such as respiratory infection) during the years following successful cancer treatment. Already in 2010, the patient was diagnosed with mild diffuse hypokinesia with LVEF of 50-55%, and in 2016 the LVEF was 54%. In the early 1990 s, first data that long‑term survivors of childhood cancer may develop irreversible, often progressive heart damage (cardiomyopathy, LV dysfunction, heart failure) and arrhythmias were published. The data of the first 15 patients with serious late clinical cardiotoxicity after therapy of childhood cancer were analysed by Steinherz et al. in 1995. Cardiac failure and arrhythmias were manifested in these series of patients from 6 to 19 years after the completion of anthracycline treatment. One out of 15 patients died, others underwent successful heart transplantation (8). In 2015, Cardinale et al. prospectively evaluated the incidence, timing of occurrence, clinical correlates, and response to heart failure therapy of cardiotoxicity in 2625 of anthracycline‑treated adult patients. Cardiotoxicity occurred in 226 (9%) of them. One hundred eighty‑three (81%) patients, were in New York Heart Association class I to II, and 43 (19%) were in class III to IV. In 9 patients, cardiotoxicity was manifested as an acute decompensated heart failure. Six of them subsequently died. In the remaining 217 patients developing cardiotoxicity, no hospitalization was needed. Heart failure therapy was initiated in all patients developing cardiotoxicity. In 40 (17% of patients), enalapril was given. The remaining 186 patients received enalapril and carvedilol or bisoprolol. Intravenous diuretics were required only in patients hospitalized for acute heart failure. Oral diuretics were added to the therapy in 43 (20% of cases). One hundred eighty‑five (82% of patients) recovered from cardiotoxicity, of those 25 (11% of patients) achieved full recovery, and 160 (71% of patients) achieved partial recovery (11).
Regarding cardiotoxicity, there is no safe cumulative dose of anthracycline. According to renowned experts in the field of cardio‑oncology lifelong follow‑up of childhood cancer survivors treated with anthracyclines is recommended. The most widely known are the recommendations of the Children’s Oncology Group (drawing from evidence‑based medicine) for the long‑term follow‑up of patients treated in childhood and under the age of 25 (5). According to these recommendations, the patient should be clinically examined annually after the completion of anthracycline treatment. At the same time, his blood pressure should be monitored annually and he should also be monitored by echocardiography (or using other imaging methods) regularly, according to the dose of doxorubicin (or equivalent dose of other anthracycline), age at time of diagnosis and chest radiotherapy as shown in Table 2. ECG also focusing on QTc interval should be performed after the completion of potential cardiotoxic treatment and later as needed. At the same time, the patient should be instructed on healthy lifestyle and aggressive management of cardiovascular risk factors. Any cancer patient under age of 5 years is considered to be at risk of cardiotoxicity when starting chemotherapy included anthracyclines. Our patient in the present article underwent cardiological examination in 2010, before the onset of cardiological symptoms, with the finding of diffuse hypokinesis and a slightly reduced LV ejection fraction of 50%. Twenty‑seven years after the completion of chemotherapy, the patient develops manifestations of heart failure and is diagnosed with sinus tachycardia and numerous ventricular extrasystoles. There is no relevant evidence for acute viral myocarditis. This patient should have been monitored annually or every other year. With regard to the type of anticancer therapy and the development of dilated cardiomyopathy, late anthracycline toxicity is considered to be the aetiology of myocardial dysfunction in our patient. Endomyocardial biopsy was not indicated. The patient responded auspiciously to routine treatment of heart failure and we assumed that it would not provide information that would affect further treatment procedure. He is currently stabilized, but with a low LV ejection fraction of 30% (based on echocardiography in October 2019). After assessing the prognostic markers (good exercise tolerance, NYHA class II, improvement of LV ejection fraction, almost normal level of NT‑proBNP), the short‑term prognosis is considered good, and so the conservative treatment procedure is continued. The patient met the criteria for primary prevention of sudden cardiac death and was implanted with the ICD. Due to the narrow QRS complex on the ECG, cardiac resynchronization therapy (CRT) was not indicated. Heart transplantation is not excluded to be considered in the future. However, the presence of CADASIL syndrome may prevent the transplantation (12, 13). CADASIL syndrome is associated with possible serious neurological and psychiatric dysfunction at a young age. Authors Lesnik Oberstein et al. published a study on the possible association between CADASIL and myocardial infarction in 2013. In this study, NOTCH3 mutation was confirmed in 41 individuals. Thirty‑two of the 41 mutation carriers had neurological symptoms, ranging from 1 transient ischemic attack to multiple strokes and cognitive decline. The evidence of myocardial infarction was found in 10 of 41 (24.4%) mutation carriers, 3 had a history of acute myocardial infarction and current Q/QS ECG abnormalities, 2 had a history of acute myocardial infarction but no current Q/QS abnormalities, and 5 had current Q/ QS abnormalities without a history of acute myocardial infarction (silent myocardial infarction) (14).
Tab. 2. Recommended frequency of echocardiographic examinations (according Children´s Oncology Group, 2018) 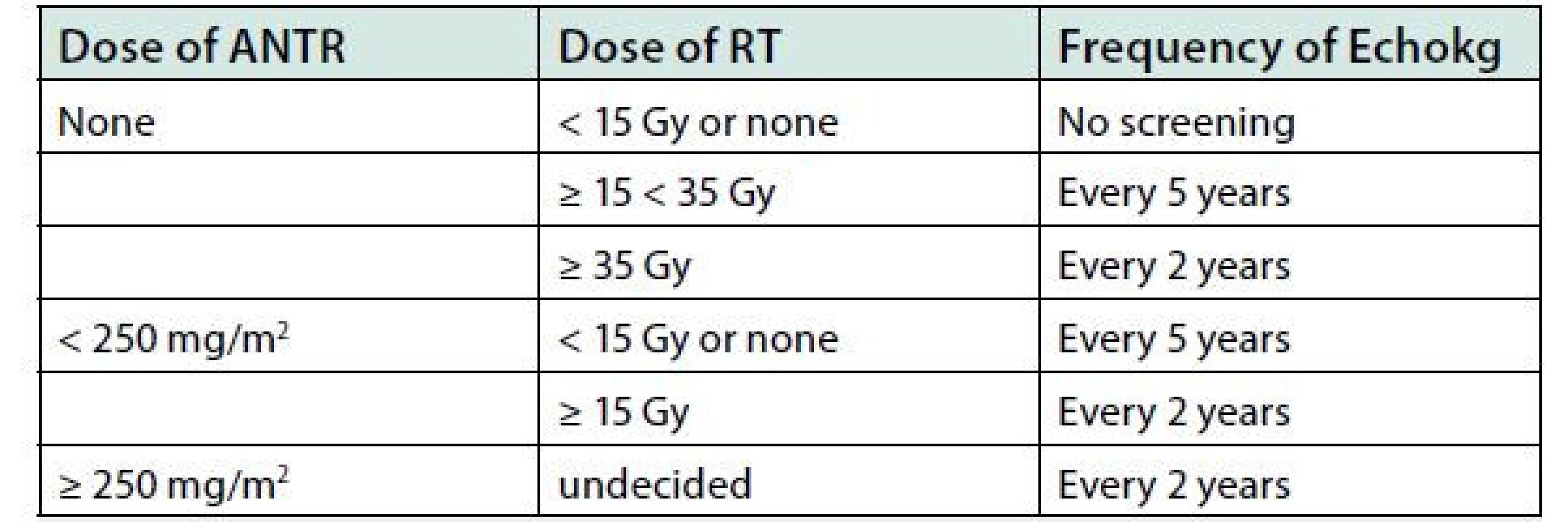
Abbreviations: ANTR, anthracycline; RT, radiotherapy; Echokg, Echocardiography In addition, the aetiology of restrictive ventilation disorder needs to be dealt with. It may involve paresis of the left diaphragm, which was noted by auscultation findings on admission and the chest X‑ray. Symptoms of late anthracycline toxicity may also include arrhythmias. (2) In case of our patient we found frequent premature ventricular contractions (PVCs). PVCs may contribute to LV dysfunction and manifestation of heart failure. Interventional radiofrequency ablation therapy is currently available and can affect the ventricular ectopy substrate and eliminate or significantly reduce PVCs. As the number of ventricular extrasystoles decreased and LVEF improved, the intervention was indicated. The present case is similar to another 4-year old patient (published by us in 2010) who was treated with standard doses of chemotherapy containing cardiotoxic daunorubicin (at a cumulative dose of 375 mg/m2), mitoxantrone, and with an allogeneic bone marrow transplantation. Twelve years after the diagnosis of acute myeloid leukemia, and following a viral infection of an unknown cause, he developed symptoms of heart failure. Severe dilated cardiomyopathy; and severe, left ventricular dysfunction with ejection fraction of 12% were found on echocardiography. The patient required a heart transplant 19 years after the diagnosis of leukemia at the age of 23 (15).
Conclusion
Late cardiac consequences after anthracycline therapy may become a complicated therapeutic problem even many years after successful anticancer therapy.
Although the treatment of heart damage is constantly improving, long‑term follow‑up of high‑risk cancer survivors should focus on subclinical cardiotoxicity before the onset of such complications, in particular on possible progressive left ventricular dysfunction, by regular monitoring of levels of serum cardiac biomarkers and using of modern imaging methods. If the cardiac dysfunction has already progressed into a heart failure, no treatment guarantees a complete restoration of the heart function. In order to better understand the pathogenesis and dynamics of late cardiovascular complications after anticancer treatment and the possibilities of its primary and secondary prevention, further randomized studies are necessary.
This publication was supported in part by a grant from Ministry of Education, Science, Research and Sport of the Slovak Republic, VEGA 01/0738/21 (administrative financial support).
KORESPONDENČNÍ ADRESA AUTORA:
MUDr. Xénia Faktorová
Interná klinika SZU, Univerzitná Nemocnica – Nemocnica svätého Michala, a.s. Satinského 1, 833 08 Bratislava
Cit. zkr: Vnitř Lék 2022;68(1):E22-E26
Článek přijat redakcí: 29. 11. 2021
Článek přijat po recenzích: 24. 1. 2022
Zdroje
1. Bates JE, Howell RM, Liu Q et al. Therapy‑Related Cardiac Risk in Childhood Cancer Survivors: An Analysis of the Childhood Cancer Survivor Study. J Clin Oncol 2019;37(13):1090 - 1101. DOI: <http://doi.org/10.1200/JCO.18.01764>.
2. Mulrooney DA, Hyun G, Ness KK et al. Major cardiac events for adult survivors of childhood cancer diagnosed between 1970 and 1999: report from the Childhood Cancer Survivor Study cohort. BMJ 2020;368:l6794. DOI: <https://doi:10.1136/bmj.l6794>.
3. Armenian SH, Hudson MM, Mulder RL et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2015;16(3):123-136. DOI: <https://doi:10.1016/S1470-2045(14)70409-7>.
4. Haddy N, Diallo S, El‑Fayech C et al. Cardiac Diseases Following Childhood Cancer Treatment: Cohort Study. Circulation 2016;133(1):31-38. DOI: <https://doi:10.1161/CIRCULATIONAHA.115.016686>.
5. Children’s Oncology Group. Data from October 2018. Long‑Term Follow‑Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 5. 0. Monrovia, CA: Children’s Oncology Group. [2021-02-28]. Retrieved from: <https://www.survivorshipguidelines.org>.
6. Hilfiker‑Kleiner D, Ardehali H, Fischmeister R et al. Late onset heart failure after childhood chemotherapy. Eur Heart J 2019;40(10):798-800. DOI: <http://doi.org/10.1093/eurheartj/ehz046>.
7. Scully RE, Lipshultz SE. Anthracycline cardiotoxicity in long‑term survivors of childhood cancer. Cardiovasc Toxicol 2007;7(2):122-128. DOI: <https://doi:10.1007/s12012-007-0006-4>.
8. Simbre VC, Duffy SA, Dadlani GH et al. Cardiotoxicity of cancer chemotherapy: implications for children. Paediatr Drugs 2005;7(3):187-202. DOI: <https://doi:10.2165/00148581-200507030-00005>.
9. Steinherz LJ, Steinherz PG, Tan C. Cardiac failure and dysrhythmias 6-19 years after anthracycline therapy: a series of 15 patients. Med Pediatr Oncol 1995;24(6):352-361. DOI: <https://doi:10.1002/mpo.2950240604>.
10. Chen Y, Chow EJ, Oeffinger KC et al. Traditional cardiovascular risk factors and individual prediction of cardiovascular events in childhood cancer survivors. J Natl Cancer Inst 2020;112(3):256-265. DOI: <https://doi:10.1093/jnci/djz108>.
11. Cardinale D, et al. Early Detection of Anthracycline Cardiotoxicity and Improvement with Heart Failure Therapy. Circulation 2015;131(22):1981-1988. DOI: <https://doi:10.1161/CIRCULATIONAHA.114.013777>.
12. Hines MR, Mulrooney DA, Hudson MM et al. Pregnancy‑associated cardiomyopathy in survivors of childhood cancer. J Cancer Surviv 2016;10(1):113-121. DOI: <https://doi:10.1007/s11764-015-0457-8>.
13. Wang J, Li J, Kong F et al. Bipolar II disorder as the initial presentation of CADASIL: an underdiagnosed manifestation. Neuropsychiatr Dis Treat 2017;13 : 2175-2179. DOI: <https://doi:10.2147/NDT.S142321>.
14. Lesnik Oberstein SA, Jukema JW, Van Duinen SG et al. Myocardial Infarction in Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL). Medicine 2003;82(4):251-256. DOI: <https://doi:10.1097/01.md.0000085054.63483.40>.
15. Urbanova D, Bubanska E, Hrebik M, Mladosievicova B. Heart transplant in a childhood leukaemia survivor: a case report. Exp Clin Transplant 2010;8(1):79-81.
Štítky
Diabetológia Endokrinológia Interné lekárstvo
Článok vyšiel v časopiseVnitřní lékařství
Najčítanejšie tento týždeň
2022 Číslo E-1- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Rizikové období v léčbě růstovým hormonem: přechod mladých pacientů k lékařům pro dospělé
- Statinová intolerance
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Zanechání kouření a riziko diabetes mellitus 2. typu
- Etické konotace provádění klinických hodnocení léčivých přípravků během pandemie onemocnění covid-19
- Možnosti cvičenia v liečbe ankylozujúcej spondylitídy
- Advanced heart failure and cardiac arrhythmia in a young adult survivor of childhood cancer
- Vyšetření renální funkce v praxi
- Muž s dýmkou, prof. MUDr. Pavel Klener, DrSc., se v dubnu 2022 dožívá 85 let
- Vnitřní lékařství
- Archív čísel
- Aktuálne číslo
- Iba online
- Informácie o časopise
Najčítanejšie v tomto čísle- Vyšetření renální funkce v praxi
- Možnosti cvičenia v liečbe ankylozujúcej spondylitídy
- Zanechání kouření a riziko diabetes mellitus 2. typu
- Advanced heart failure and cardiac arrhythmia in a young adult survivor of childhood cancer
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



