-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
A primary cutaneous carcinosarcoma of the retro auricular region, how to treat and literature review
Authors: C. Arkaz 1,2; J. Pauwels 2; K. Wetzels 3; B. Cambier 1
Authors place of work: Faculty of Medicine, University of Antwerp, Antwerp, Belgium 2; Department of Pathology, Sint-Blasius Hospital, Dendermonde, Belgium 3
Published in the journal: ACTA CHIRURGIAE PLASTICAE, 65, 3-4, 2023, pp. 140-146
doi: https://doi.org/10.48095/ccachp2023140Introduction
Carcinosarcoma (CS) is a rare malignant blend of epithelial and heterogenous mesenchymal tissues [1]. Though most commonly described in visceral organs such as breast, urinary bladder, uterus, liver, colon and lungs, we present a case of a primary cutaneous tumour [2]. Typically, the cutaneous variant is found in sun-damaged skin, such as the face and scalp. They often present as a rapidly growing, painless mass or nodule on the skin. Its nodular, often ulcerated, appearance alone is not enough to distinguish cutaneous CS (CCS) from other skin cancers, with basal cell carcinoma (BCC) and spinocellular carcinoma being the most plausible. When confronted with CCS, the possibility of metastasis of a visceral CS should always be excluded, as this is associated with a much poorer prognosis [1,3–5]. Diagnosis requires histopathologic sampling and an immunohistochemical panel of the tissue following clinical suspicion [3,5]. Prognosis depends mostly on the aggressivity of the epithelial component, tumour size, age at presentation, involvement of lymph nodes and metastatic lesions [1,6,7].
The first CCS was described in 1972 by Dawson et al. and since then, only 181 were reported in the literature [8]. However, this might be underreported due to a lack of knowledge, variation in tissue sampling and a broad spectrum of clinical and histological phenotypes [9]. Many terms are used to describe this type of tumour, including CS, (biphasic) sarcomatoid carcinoma, metaplastic carcinoma, pseudosarcoma, malignant mixed tumour, and spindle cell carcinoma [10,11].
Case report
A Caucasian 84-year-old female patient was referred to the plastic surgery department by her dermatologist. Her medical history showed a left-sided mastectomy with axillar clearance due to an invasive ductal carcinoma in 2005. The surgery was followed by six cycles adjuvating chemotherapy according to a CMF-protocol, postoperative radiotherapy up to 50 Gy and Nolvadex. Aromasin was subjectively not well supported. We withheld no further systemic diseases nor chronic medication.
The patient presented herself with a retroauricular skin tumour on the right side. A biopsy taken by her dermatologist prior to referral, identified this lesion as morpheaform BCC without perineural invasion. According to the pathology report, the biopsy nearly completely removed the lesion. Referral was thus postponed because of the COVID-19 pandemic. Although it was 18 months after the procedure, a tumour with an accelerated growth pattern appeared at the same location.
Fig. 1. Iconography pathology HE and IHC with comments. 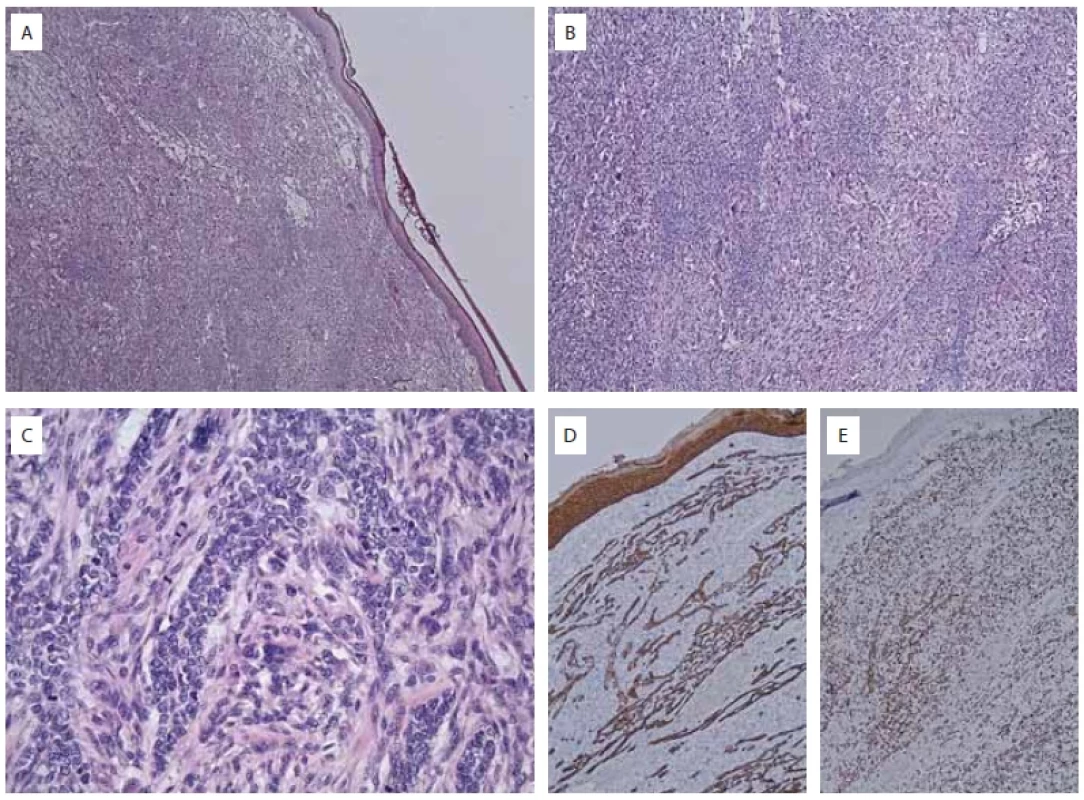
A) epidermis and dermis entirely involved by tumor (HE, ×25); B) highly anaplastic process growthing in sheets and bundles (HE, ×50); C) highly mitotically active plump and spindled tumor cells with striking anisokaryosis and aberrant mitotic fi gures and some multinuclear giant cells (HE, ×200); D) diff use expression of cytokeratine 5/6 in tumor cells and overlying epidermis (IHC, ×50); E) diff use and strong aberrant overexpression of p53 in almost all tumor cells (IHC, ×50). Upon examination we saw a keloid-like nodular tumour. Raised about 2.4 cm from the skin surface, 3 cm in diameter. The lesion had a purple red colour with a small central zone of necrosis and dimpling.
Removal was performed under local anaesthesia with intraoperative cryosection. Due to macroscopic aberrations, a second deep section specimen was sent to pathology. Cryosection indicated a clear-cutting margin for invasive carcinoma. Primary closure was impossible because of the defect size and was hence performed with a V-Y advancement flap.
All samples were fixed in 10% formalin, routinely processed and embedded in paraffin. Then, 4 mm thick sections were stained with haematoxylin and eosin. After 5 days, we received the pathology report as follow: the large nodular lesion macroscopically described corresponds microscopically to a superficially ulcerated, high-grade neoplastic lesion growing in the form of capricious, interdigitating nests accompanied by an atypical stromal reaction. These nests are composed of polygonal amphophilic tumour cells with important anisokaryosis and high mitotic activity of atypical mitosis figures. No clear features of squamous differentiation. There is significant atypia of the stromal phase: quite important nuclear atypia and mitotic activity. In this stromal component we also see ectopic bone formation. The lesion reaches focal to the deep, inked plane of cut, indicating transection of the plane of cut. No lymphovascular or blood vessel invasion was reported. This pattern corresponds to a CS of the skin with a focal deep positive section plane. In addition, the deep section specimen pathology report showed no indication for tumoral invasion or aberrations, so a clear section plane was obtained.
Furthermore, sections from each case were subjected to appropriately controlled immunohistochemical reactions employing cytokeratin AE1/AE3, epithelial membrane antigen (EMA), p53, p63, vimentin, desmin and S-100 protein. Immunohistochemically, the stromal component was diffusely and strongly positive for vimentin, while the epithelial component showed strong positivity for p63. CD34 was only positive in the endothelium (Fig. 1).
Post-operative wound healing was favourable and dermatological follow-up showed no recurrence or progressive disease in this patient 36 months after complete excision.
Discussion
Study selection
A manual electronic search of the PubMed Medline and Web of Science Core Collection databases, encompassing all included reports until the end of November 2022, was performed to identify reports of CCS. The following search query was used limited to citation titles and abstracts: cutaneous carcinosarcoma or carcinosarcoma of the skin and skin or subcutaneous or cutaneous or superficial. References of selected articles were also reviewed to identify potential additional cases (Scheme 1).
Aetiology
Although histogenesis remains obscure, several hypotheses try to explain the development of carcinosarcomas. The monoclonal hypothesis states a divergent differentiation of a single totipotential progenitor cell in to malignant epithelial and mesenchymal populations [6,12]. In the multiclonal hypothesis, the tumour originates from two separate progenitor cells [13,14]. Another hypothesis says the CS is formed by a collision of two unique neoplasms [15]. The notion of monoclonality in CS, as suggested by immunohistochemical, ultrastructural, and molecular genetic studies, is nowadays accepted [7,16,17].
Risk factors described in the literature consist of older age (after the fifth decade), male sex and chronically sun-exposed skin areas [1,18–20]. A few case reports identify immunosuppression as a risk factor for CCS, as it is for conventional BCC [3,21]. Repeated trauma can also be a contributing factor as two cases showed non-healing scalp masses secondary to trauma [22]. Lim et al. presented a case in which an orthopaedic surgeon, who had regularly been exposed to radiation from C-arm fluoroscopy for 20 years, developed CCS on his hand [23].
The presence of p53 mutations in both epithelial and mesenchymal tumour components caused by UV radiation might be a key in neoplastic transformation [6,10,24,25]. Studies on tumour genetics found in both epithelial and mesenchymal components strong immunohistochemical staining for p53, correlating with p53 protein mutations [16], suggestive for a common origin and supporting the monoclonal hypothesis in which the sarcomatous component might be seen as a result of dedifferentiation [17,26]. Identical mutations of this tumour suppressor gene p53 have been found in visceral carcinosarcomas in both components of the malignancy [27]. In tumours where the epithelial component is composed of BCC mutations in PTCH1, p53, p63 and p13 genes are commonly found, whereas in SCC mainly has p53 point mutations [7].
Diagnosis
CCS is typically found in sun-damaged skin, most often on the face or scalp. Even well-experienced clinicians can’t distinguish a CCS from other skin cancers due to its nodular exophytic appearance, often with an ulceration. To ensure accurate diagnosis, it is crucial to identify the biphasic nature of this tumour, which involves the presence of malignant epithelial and mesenchymal components. Therefore, meticulous tissue sampling is necessary.
As proposed by Muller et al. three diagnostic criteria can be utilized for primary CCS: 1) a bimodal neoplasm consisting both malignant epithelial and mesenchymal elements confirmed by histological examination and immunohistochemistry (IHC); 2) exclusion of distant metastasis, especially from the genitourinary tract and lungs, and of true collision neoplasms, defined as two different neoplasms coexisting in one biopsy specimen; and 3) a solid coherent tumour with exclusion of reactive sarcomatous changes in the stroma [3].
CCS are roughly classified as being epidermal, those with squamous cell carcinoma (SCC) or BCC, or being adnexal, including malignant pilomatrixoma, spiradenocarcinoma, trichoblastic carcinoma, porocarcinoma and Merkel cell tumour [6,14,16,28]. The mesenchymal component consists of undifferentiated spindle and pleomorphic cells with nuclear atypia, necrosis and atypical mitotic figures. Malignant heterologous mesenchymal elements might be present, such as osteosarcoma or chondrosarcoma. Rarely skeletal muscle, smooth muscle, myofibroblastic, fibrosarcomatous, or angiosarcomatous differentiation have been reported [1,19,29]. It is possible for various subtypes to coexist within a single tumour. A close-knit transition zone can be found in between the two cell populations.
The histomorphologic biphasic cell populations have a different immunoreactivity with epithelial components generally being positive for cytokeratin (CK) markers, mainly CK AE1/AE3, or p63 and MNF116 if cells are poorly differentiated, and mesenchymal components possibly showing reactivity for vimentin, actin, CD10, CD34, CD68 and CD99, depending on differentiation of the tumour [3,18,30]. In BCC’s positivity for BerEP4 and Bcl-2 has also been observed [23]. Furthermore, overexpression of p53 has been reported in both epithelial and mesenchymal components [16,23].
Survival and prognosis
In CCS, survival strongly depend on the epithelial component and seems not to be influenced by the type or proportion of the sarcomatous component [22]. Epidermal derived CS present on sun-damaged skin in older males (mean age 72 years) with a 70% 5-year disease-free survival, as opposed to adnexal derived CS which occur in younger patients (mean age 58 years) with a long-standing nodule with recent rapid growth and a 25% 5-year disease-free survival [6,11,19]. Survival rates of CCS are lower than in conventional BCC and SCC which can have a 5-year survival rate above 95%. With a 5-year survival rate of 99% localized CCS have a great prognosis [31]. When facing a CCS, it is crucial to rule out the possibility of a visceral CS metastasis, as it is linked to a significantly worse prognosis [1,3–5].
Multiple studies also identified factors such as larger tumour size, positive regional lymph nodes, metastatic lesions, advanced age at presentation and rapid tumour growth contributing to an aggressive clinical course [9,32,33]. Metastatic disease in basal cell CS is about 2% similar to large BCC (> 3 cm) [3,18,21]. In comparison, squamous cell CS or adnexal CS tend to have higher metastatic rates, ranging from 12 to 50% [6,9,19]. The absence of intraoperative margin control during surgery can lead to higher recurrence and metastasis rates due to the tumour’s characteristics [5].
Treatment
CCS occur in a variety shapes and sizes; hence management should be based on clinical and histological features [19]. Guidelines regarding treatment and follow-up have not yet been established. However, since data concerning prognosis is limited, complete surgical excision of the CCS is the treatment of choice, as there are reports of aggressive recurrence in cases without complete excision; moreover, chest imaging should be considered [1,7,34]. With regards to definitive margins for the excision of primary cutaneous manifestations there is no consensus; as the margins in previous case reports vary from 0.5 to 3.5 cm [1,12,18,20,35,36]. Alternatively, Mohs micrographic surgery (MMS) has been reported several times as a treatment option for CCS [1,5]. It is likely beneficial for large and complex tumours since it aims to limit the resection of heathy tissue while ensuring intraoperative analysis of all surgical margins [37,38]. Some authors claim MMS is the treatment of choice in CCS with a cure rate of ≥ 98%; in opposition to a recurrence rate of 33% in cases without surgery [3,25]. Therefore, careful monitoring of the patient is necessary if MMS is not conducted.
The place of adjuvant radiotherapy or chemotherapy in the treatment of CCS is uncertain as the paucity of cases shows no evidence supporting therapeutic benefit [9,12,16,21,25,39]. One patient with a CCS penetrating the scalp declined surgical excision and was successfully treated solely with radiotherapy [24]. In three cases, an amputation of the hand or fingers was the preferred technique for tumour management [23,33,35].
CCS of the basaloid subtype should be managed as a high-risk BCCs and perform appropriate margin control post-excision or MMS [22,39]. While surgical excision with clear margins is typically curative in trichoblastic CCS, chemotherapy is worth considering in metastatic disease [40].
Whether wide local excision or MMS is used, the resulting defects can be large and in obviously visible locations. Dermatologists should therefore consider referral to a plastic surgeon for adequate reconstruction of the defect. Reconstruction should be according to traditional teaching in plastic and reconstructive surgery using the reconstructive ladder, starting with healing by secondary intent up to free-tissue transfer, depending on the location, size of the defect, and extent of exposed tissue and type. An adequate reconstruction may be as important as the primary resection of the skin cancer in protection of exposed vital structures [22].
In the literature, 19 cases of CCS were reported where a reconstruction technique was employed to close the resulting skin defect after excision of the tumour. A single case report presents wound closure by secondary intention [41]. Skin grafting was addressed in twelve skin defects, of which two in combination with other reconstructive techniques [7,9,23,29,32,42–47]. Local flaps were the preferred treatment in four cases, i.e. a Hughes flap, a cervicofacial rotation flap or an orticochea flap [22,47–49]. Another report discussed a regional flap reconstruction using a reverse sural flap [29]. Tissue expansion was applied in two cases [20,50]. One case described a free tissue flap, as an anterolateral thigh flap was used to cover up a large defect on the scalp [22].
The recommended follow-up period is 5 to 10 years, with initial intervals of 3 to 6 months. After completing the follow--up, patients should be advised to seek medical attention when early signs of recurrence appear [51].
Conclusion
In conclusion, CCS is a rare and aggressive malignancy characterized by the presence of both malignant epithelial and mesenchymal components. Risk factors associated with CCS include older age, male sex, chronically sun-exposed skin, immunosuppression, repeated trauma, and exposure to radiation. Genetic studies have identified mutations in p53 as a key factor in neoplastic transformation.
Accurate diagnosis of CCS requires histological examination and immunoreactivity of epithelial and mesenchymal components to specific markers helps differentiate CCS from other skin cancers. Prognosis and survival in CCS are influenced by the epithelial component, with epidermal-derived CCS having better outcomes than adnexal-derived CCS. Factors such as larger tumour size, positive regional lymph nodes, metastatic lesions, advanced age, and rapid tumour growth contribute to a more aggressive clinical course.
The primary treatment for CCS is complete surgical excision with clear margins, although the optimal margin size is still debated. We believe MMS is the best method for the excision of CCS, as it has shown promising results in limiting resection of healthy tissue and reducing recurrence rates. The role of adjuvant radiotherapy or chemotherapy in CCS treatment remains uncertain due to limited evidence.
Reconstruction of the resulting defects after excision of CCS is essential, especially when located in visible areas of involving vital structures. Dermatologists may consider referring patients to plastic surgeons for appropriate reconstruction techniques based on the location, size and extent of the defect. Follow-up should be conducted for 5 to 10 years, with regular intervals to monitor for recurrence.
Overall, CCS represents a challenging malignancy that requires a multidisciplinary approach for accurate diagnosis, effective treatment and optimal reconstruction. Further research is needed to improve our understanding of the aetiology, prognosis and optimal management strategies for CCS.
Roles of authors
Coskun Arkaz – concept, design, data acquisition, data analysis, manuscript preparation, manuscript editing;
Jasper Pauwels – literature search, data acquisition, data analysis, manuscript preparation;
Kevin Wetzels – pathology report and comments, manuscript editing, manuscript review.
Bernard Cambier – design, manuscript preparation, manuscript editing, manuscript review.
Conflict of interest: The authors declare that they have no conflict of interest.
Declaration of patient consent to participate and publish: The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship: The corresponding authors are not recipients of any research scholarship, grant or funding for this work.
Zdroje
1. Kwak HB., Park J., Kim HU., et al. Cutaneous carcinosarcoma: a clinicopathologic and immunohistochemical analysis of 11 Korean cases. J Korean Med Sci. 2019, 34 (1): e5.
2. Ram R., Saadat P., Peng D., et al. Primary cutaneous carcinosarcoma. Ann Clin Lab Sci. 2005, 35 (2): 189–194.
3. Müller CS., Pföhler C., Schiekofer C., et al. Primary cutaneous carcinosarcomas: a morphological histogenetic concept revisited. Am J Dermatopathol. 2014, 36 (4): 328–339.
4. Gungorduk K., Ozdemir A., Ertas IE., et al. Adjuvant treatment modalities, prognostic predictors and outcomes of uterine carcinosarcomas. Cancer Res Treat. 2015, 47 (2): 282–289.
5. García-Souto F., Pereyra-Rodriguez JJ., Cabrera-Perez R., et al. Primary cutaneous carcinosarcoma: clinical, histological, and immunohistochemical analysis of eight cases. Int J Dermatol. 2021, 60 (1): 93–98.
6. Tran TA., Muller S., Chaudahri PJ., et al. Cutaneous carcinosarcoma: adnexal vs. epidermal types define highand low-risk tumors. Results of a meta-analysis. J Cutan Pathol. 2005, 32 (1): 2–11.
7. Bigby SM., Charlton A., Miller MV., et al. Biphasic sarcomatoid basal cell carcinoma (carcinosarcoma): four cases with immunohistochemistry and review of the literature. J Cutan Pathol. 2005, 32 (2): 141–147.
8. Dawson EK. Carcino-sarcoma of the skin. J R Coll Surg Edinb. 1972, 17 (4): 243–246.
9. Syme-Grant J., Syme-Grant NJ., Motta L., et al. Are primary cutaneous carcinisarcomas underdiagnosed? Five cases and a review of the literature. J Plast Reconstr Aesthet Surg. 2006, 59 (12): 1402–1408.
10. Gómez-Espejo C., Herrera-Sabal A., Ríos-Martín JJ., et al. Basal cell carcinoma with sarcomatoid features (sarcomatoid carcinoma): report of a case and review of the literature. J Dermatol. 2003, 30 (7): 543–549.
11. Ndukwe CO., Chiemeka ME., Menkiti FE., et al. Primary carcinosarcoma of the skin in an African albino: case report and review of literature. Oman Med J. 2022, 37 (5): e417.
12. Liaw TY. Primary cutaneous sarcomatoid carcinoma presenting as a rapidly-growing and ulcerative tumor of the skin. Kaohsiung J Med Sci. 2017, 33 (6): 315–317.
13. Moore DJ., Powis G., Richardson RL., et al. Topical chemotherapy of intradermal Walker 256 carcinosarcoma with diaziquone and doxorubicin in the rat. Cancer Res. 1985, 45 (11 Pt 1): 5466–5472.
14. Patel NK., McKee PH., Smith NP., et al. Primary metaplastic carcinoma (carcinosarcoma) of the skin. A clinicopathologic study of four cases and review of the literature. Am J Dermatopathol. 1997, 19 (4): 363–372.
15. Luong TMH., Akazawa Y., Mussazhanova Z., et al. Cutaneous pilomatrical carcinosarcoma: a case report with molecular analysis and literature review. Diagn Pathol. 2020, 15 (1): 7.
16. Rose RF., Merchant W., Stables GI., et al. Basal cell carcinoma with a sarcomatous component (carcinosarcoma): a series of 5 cases and a review of the literature. J Am Acad Dermatol. 2008, 59 (4): 627–632.
17. Loh TL., Tomlinson J., Chin R., et al. Cutaneous carcinosarcoma with metastasis to the parotid gland. Case Rep Otolaryngol. 2014, 2014 : 173235.
18. Zbacnik AP., Rawal A., Lee B., et al. Cutaneous basal cell carcinosarcoma: case report and literature review. J Cutan Pathol. 2015, 42 (11): 903–910.
19. Clark JJ., Bowen AR., Bowen GM., et al. Cutaneous carcinosarcoma: a series of six cases and a review of the literature. J Cutan Pathol. 2017, 44 (1): 34–44.
20. Wollina U., Riedel I., Abushika MR., et al. Giant pendulous carcinosarcoma – squamous cell carcinoma-type – of the leg – a case report and review of the literature. Open Access Maced J Med Sci. 2018, 6 (1): 112–114.
21. Davis K., Whale K., Tran S., et al. A rare case of cutaneous basal cell carcinosarcoma in an immunosuppressed patient. Pathology. 2020, 52 (2): 267–268.
22. Song EY., Wallace SJ., Sheikh H., et al. Cutaneous carcinosarcoma: a small case series and review of the literature of a rare skin tumor. Cureus. 2020, 12 (8): 10.
23. Lim Y., Byun HJ., Park CS., et al. Primary cutaneous carcinosarcoma developing after chronic C-arm radiation exposure. JAAD Case Rep. 2018, 4 (2): 126–128.
24. Cervoni G., Steffes WE., Kobraei KB., et al. Exophytic scalp tumor the diagnosis: primary cutaneous carcinosarcoma. Cutis. 2016, 97 (4): E9–E11.
25. Ruiz-Villaverde R., Aneiros-Fernández J. Primary cutaneous carcinosarcoma: a cutaneous neoplasm with an exceptional presentation. Sultan Qaboos Univ Med J. 2018, 18 (1): e114–e115.
26. Kiuru M., McDermott G., Coit DC., et al. Basal cell carcinosarcoma with PTCH1 mutations in both epithelial and sarcomatoid primary tumor components and in the sarcomatoid metastasis. Am J Surg Pathol. 2014, 38 (1): 138–142.
27. Ansari-Lari MA., Hoque MO., Califano J., et al. Immunohistochemical p53 expression patterns in sarcomatoid carcinomas of the upper respiratory tract. Am J Surg Pathol. 2002, 26 (8): 1024–1031.
28. Tan KB., Murali R., Karim RZ., et al. Merkel cell carcinoma with fibrosarcomatous differentiation. Pathology. 2008, 40 (3): 314–316.
29. Agostini T., Mori A., Leporatti G., et al. Cutaneous carcinosarcoma: report of a case with myofibroblastic sarcomatous component. Dermatol Surg. 2008, 34 (3): 418–422.
30. Patterson JW. Tumors of the epidermis. In: Wheedon‘s Skin Pathology. Elsevier 2016 : 784–835.
31. Blake PW., Bradford PT., Devesa SS., et al. Cutaneous appendageal carcinoma incidence and survival patterns in the United States: a population-based study. Arch Dermatol. 2010, 146 (6): 625–632.
32. Hong SH., Hong SJ., Lee Y., et al. Primary cutaneous carcinosarcoma of the shoulder: case report with literature review. Dermatol Surg. 2013, 39 (2): 338–340.
33. El Harroudi T., Ech-Charif S., Amrani M., et al. Primary carcinosarcoma of the skin. J Hand Microsurg. 2010, 2 (2): 79–81.
34. Kim C., Brown A., Osipov V. Trichoblastic carcinosarcoma in a 34-year-old woman with histopathologic and molecular analysis, including re-demonstration of a CDKN2A p. (R58*) mutation. J Cutan Pathol. 2021, 48 (2): 334–339.
35. Chittari K., Birnie AJ., Kulkarni KR., et al. Sarcomatoid carcinoma of the hand: a clinical case with an aggressive and uncommon presentation. Clin Exp Dermatol. 2012, 37 (5): 505–508.
36. Suzuki H., Hashimoto A., Saito R., et al. A case of primary cutaneous basal cell carcinosarcoma. Case Rep Dermatol. 2018, 10 (2): 208–215.
37. Etzkorn JR., Alam M. What is mohs surgery? JAMA Dermatology. 2020, 156 (6): 716.
38. Fania L., Didona D., Morese R., et al. Basal cell carcinoma: from pathophysiology to novel therapeutic approaches. Biomedicines. 2020, 8 (11): 449.
39. Bourgeault E., Alain J., Gagné E. Primary cutaneous carcinosarcoma of the basal cell subtype should be treated as a high-risk basal cell carcinoma. J Cutan Med Surg. 2015, 19 (4): 407–411.
40. Underwood CIM., Mansoori P., Selim AM., et al. Trichoblastic carcinosarcoma: a case report and literature review. J Cutan Pathol. 2020, 47 (4): 409–413.
41. Leen EJ., Saunders MP., Vollum DI., et al. Carcinosarcoma of skin. Histopathology. 1995, 26 (4): 367–371.
42. Xu C., Ibbetson J., Yeoh TM., et al. Aggressive growth of an incompletely excised primary cutaneous basal cell carcinosarcoma on the scalp: a case report. ANZ J Surg. 2016, 86 (12): 1065–1066.
43. Okhremchuk I., Nguyen AT., Fouet B., et al. Trichoblastic carcinosarcoma of the skin: a case report and literature review. Am J Dermatopathol. 2018, 40 (12): 917–919.
44. Bakhshi GD., Wankhede KR., Tayade MB., et al. Carcino-sarcoma in a case of syringocystadenoma papilliferum: a rare entity. Clin Pract. 2012, 2 (3): e71.
45. Ishikawa M., Nakanishi Y., Yamazaki N., et al. Malignant eccrine spiradenoma: a case report and review of the literature. Dermatol Surg. 2001, 27 (1): 67–70.
46. De Francesco V., Frattasio A., Pillon B., et al. Carcinosarcoma arising in a patient with multiple cylindromas. Am J Dermatopathol. 2005, 27 (1): 21–26.
47. Konstantinidis A., Ghosh Y., Snead D., et al. Eyelid basal cell carcinoma with osteosarcomatous changes. Ophthalmic Plast Reconstr Surg. 2008, 24 (4): 322–323.
48. Venishetty N., Shah KM., Nijhawan RI. Primary cutaneous carcinosarcoma treated with mohs micrographic surgery in a hospice patient. Dermatol Surg. 2022, 48 (12): 1359–1360.
49. Scholl P., Snyder N., Patt D., et al. Pilomatrical carcinosarcoma. Otolaryngol Head Neck Surg. 2010, 143 (5 Suppl 3): 36–37.
50. Wollina U., Koch A., Schönlebe J., et al. Carcinosarcoma of skin (sarcomatoid carcinoma) – a rare non-melanoma skin cancer (case review). Georgian Med News. 2017, (263): 7–10.
51. Sangjin O., Haneul L., Sungyul L., et al. Primary cutaneous sarcomatoid carcinoma. Indian J Dermatol Venereol Leprol. 2012, 78 (5): 665.
Coskun Arkaz, MD
Faculty of Medicine and Health Sciences Antwerp University
Universiteitsplein 1
2610 Wilrijk (Antwerp)
Belgium
e-mail: Coskunarkaz@gmail.comSubmitted: 28. 8. 2023
Accepted: 7. 2. 2024Štítky
Chirurgia plastická Ortopédia Popáleninová medicína Traumatológia
Článok vyšiel v časopiseActa chirurgiae plasticae
Najčítanejšie tento týždeň
2023 Číslo 3-4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Srovnání analgetické účinnosti metamizolu s ibuprofenem po extrakci třetí stoličky
- Možnosti využití metamizolu v léčbě akutních primárních bolestí hlavy
-
Všetky články tohto čísla
- Editorial
- Diagnosis and treatment of Eagle’s syndrome and possible complications
- Scalp arteriovenous malformations – 20 years of experience in a tertiary healthcare centre
- The comparison of effectivity in breast cancer prevention between skin sparing and subcutaneous mastectomy – 20 years of experience
- Avascular necrosis of the maxilla after orthognathic surgery, a devastating complication? A systematic review of reported cases and clinical considerations
- 3D maxillofacial surgery planning – one decade development of technology
- A primary cutaneous carcinosarcoma of the retro auricular region, how to treat and literature review
- Combination of cable ties and barbed sutures for fasciotomy closure – two case reports
- Skin grafting on amputated lower limb, norepinephrine-induced ischemic limb necrosis – case report
- Abdominal wall reconstruction for extensive necrosis following abdominoplasty in a patient with subcostal scars – case report
- Acta chirurgiae plasticae
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Diagnosis and treatment of Eagle’s syndrome and possible complications
- Avascular necrosis of the maxilla after orthognathic surgery, a devastating complication? A systematic review of reported cases and clinical considerations
- 3D maxillofacial surgery planning – one decade development of technology
- Skin grafting on amputated lower limb, norepinephrine-induced ischemic limb necrosis – case report
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy




