-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Acinetobacter – serious danger FOR burn patients
Autoři: J. Babík; L. Bodnárová; K. Sopko
Působiště autorů: Clinic for Burns and Reconstructive Surgery Košice-Šaca, Slovakia
Vyšlo v časopise: ACTA CHIRURGIAE PLASTICAE, 50, 1, 2008, pp. 27-32
Introduction and Taxonomy
Acinetobacter was described for the first time by Beijerinck as Micrococcus calco-aceticus (Beijerinck 1911), while a detailed study was presented later by Baumann et al. in 1968 and Bouvet and Grimont in 1986 (2, 7).
The Acinetobacter genus is one of a number of non-fermenting aerobic gram-negative structures (1). Acb can be isolated from soil, water and raw meat samples and are part of the physiological bacterial flora of the skin, especially in the areas of the perineum, inguina and axillas (2, 3). Due to their distinct adhesive ability to epithelial cells, the process of colonization or infection of the skin, wounds, mucous membranes and upper respiratory airways gives priority to Acinetobacters in comparison with other microbes (5).
The hospital environment becomes a secondary source of contamination; technical equipment of the ICU ventilation, moisturizers, water, bronchoscopes, mattresses and pillows, all present a secondary reservoir of Acinetobacter strains (10, 11).
The ability of Acb to survive in both very dry and wet conditions, their affinity for plastic and metal materials, and their non-abrasive growth lead to failure in hygiene prophylaxis (12). In 2006 we recorded the first multi-drug-resistant Acinetobacter baumannii in 8 patients. The emergence of resistant strains has become aĘmajor problem in ICUs worldwide (1).
Patients and methods
In 2006 we studied 270 admitted patients in our burn clinic, aged between 3 months and 91 years (mean 29.4±8.7); 71% were males. The aetiology of the burn trauma was scalds (58.64%), flame (24.5%), electric burns (5.6%) and chemical agents (4.5%). Special attention was paid to all patients from ICU (34) with a record of invasive procedures, surgery and ATB treatment. The medical records of each patient were reviewed for age, sex, diagnosis, the date of the injury, admission to the ICU, length of stay in ICU, biological material and day of the first A. baumannii isolation, broad spectrum antibiotics used, topical antimicrobial treatment, presence of diagnostic and therapeutic and surgical interventions.
Bacterial culture swabs from burn wounds were taken on admission and every 48 hours a week depending on clinical status. Blood cultures were examined with use of the Bactec system (Becton Dickinson) taken with pending clinical status. Phenotype identification and isolation of A. baumannii was performed with the base standard microbiological methods. Biochemical identification was provided by BBL Crystal by Enteric/Neferm test (Becton Dickinson), and for sensitivity tests to 17 antimicrobial agents microdilution tests Bel-Miditech were used according to the norms established by the NCCLS (National Committee for Clinical Laboratory Standards).
Results
During the period 2003-2006, 1087 patients were hospitalized at the Clinic for Burns in Košice. 4152 bacterial strains were isolated from biological material, mostly bacterial swabs, blood cultures, urine, sputum etc. The 2991 most frequently isolated bacterial strains, with serious clinical impact, were collected for statistical analysis and for more detailed study (Table 1).
Tab. 1. Incidence of bacterial strains in burn department and ICU in 2006 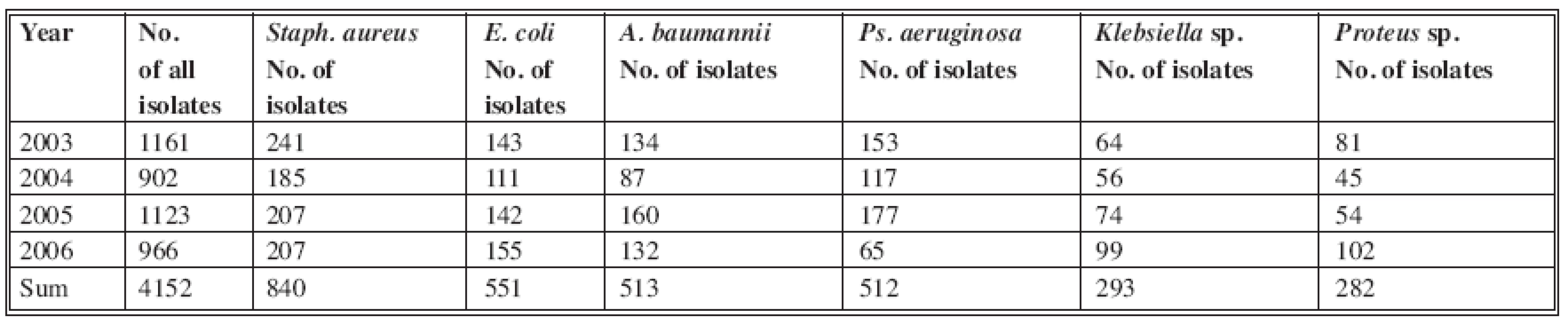
Staphylococcus aureus was isolated in 840 cases (20%) - the highest incidence. Of the gram-negative isolated bacterial strains in this period, Acinetobacter baumannii was found with 513 isolates (12%) and Pseudomonas aeruginosa with 512 isolates (12%). E. coli was the most frequently isolated one, with 551 positive cultures (12.8%) (Fig. 1). Other, small numbers of isolates (Proteus vulgaris, Morganella morganii, Enterobacter aerogenes, Enterobacter cloace, Candida albicans) were not included in the study.
Obr. 1. The most frequent isolates in Burn Department and ICU (in %) 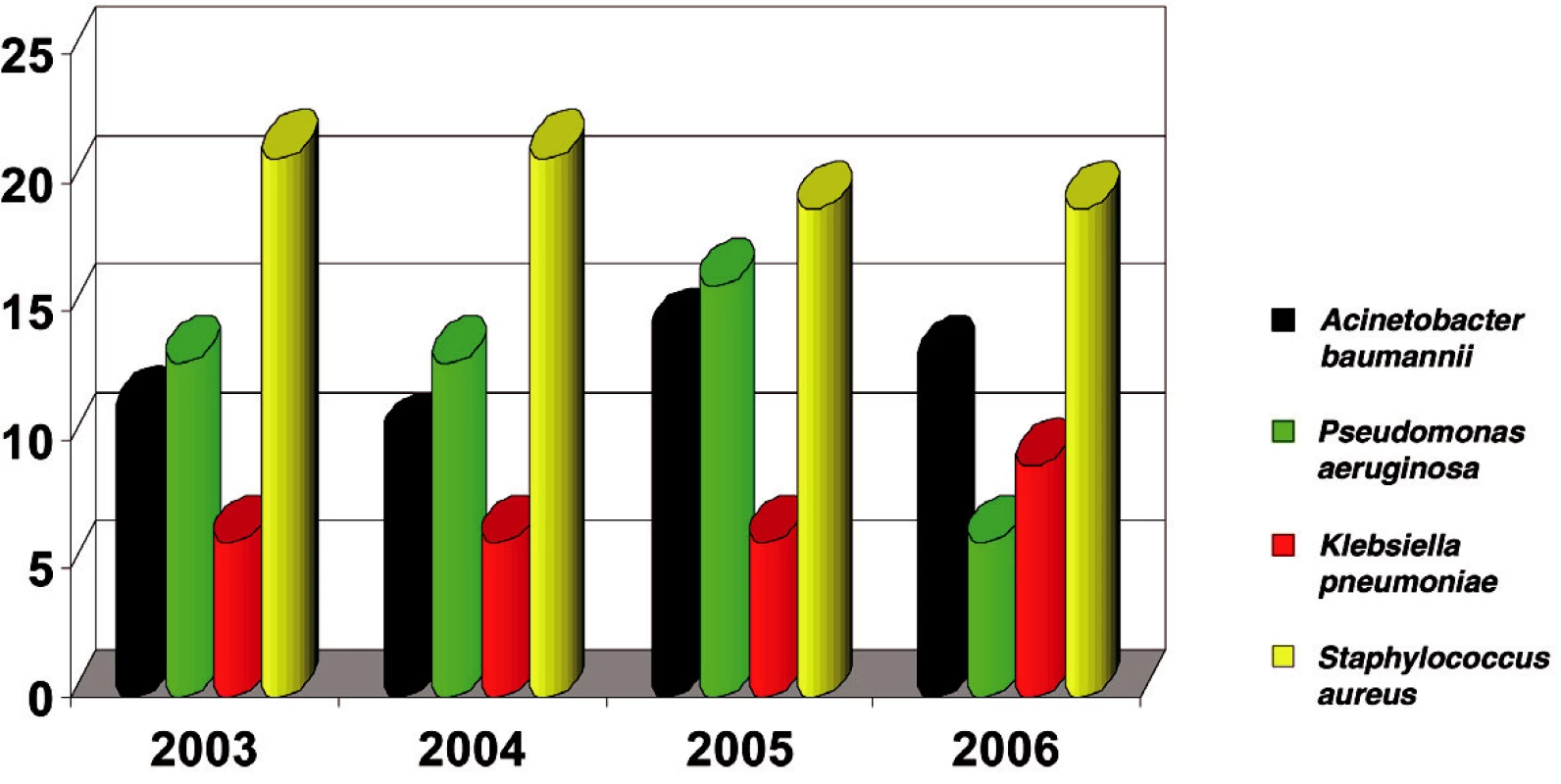
In 2003, 134 A. baumannii species were isolated with resistance to Ampicilin/Sulbactam (16 %) and Meropenem (0%) at that time; in 2006, 132 isolates significantly demonstrated increased resistance to Ampicilin/Sulbactam (up to 55%) and Meropenem (4%). The highest resistance was to Gentamycin, which increased to 90% (Table 2).
Tab. 2. Distribution of A. baumannii resistance in 2003–2006 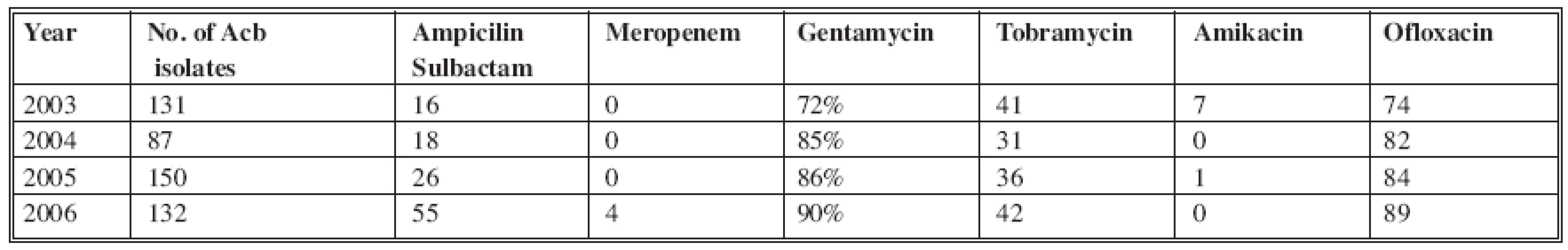
A group of 34 patients admitted to the ICU in 2006 with positive evidence of A. baumannii strains from biological material were selected for detailed study (Table 3). The group of 34 patients comprised 22 adults with a mean age of 50 years (range 20-79) and 12 children with a mean age 5 years (range 1-15): 24 patients (71%) were males and 10 (29%) were females. All of the patients were admitted to the ICU with TBSA 27% (range 3-78%). Ten patients (29%) were admitted to the ICU on the day of the injury and 24 patients (71%) were admitted to the ICU from other hospitals 48 hours post burn injury. Four patients (12%) had isolated A. baumannii from the wound swabs on the day of the admission to the ICU. After the next 48 hours, 3 patients (9%) had their first positive isolation. On average, 27 patients (79%) had positive isolation of A. baumannii from wound swabs on the sixth day after admission to the ICU.
Tab. 3. ICU patients with positive isolations of Acinetobacter complex strains from biological material in 2006 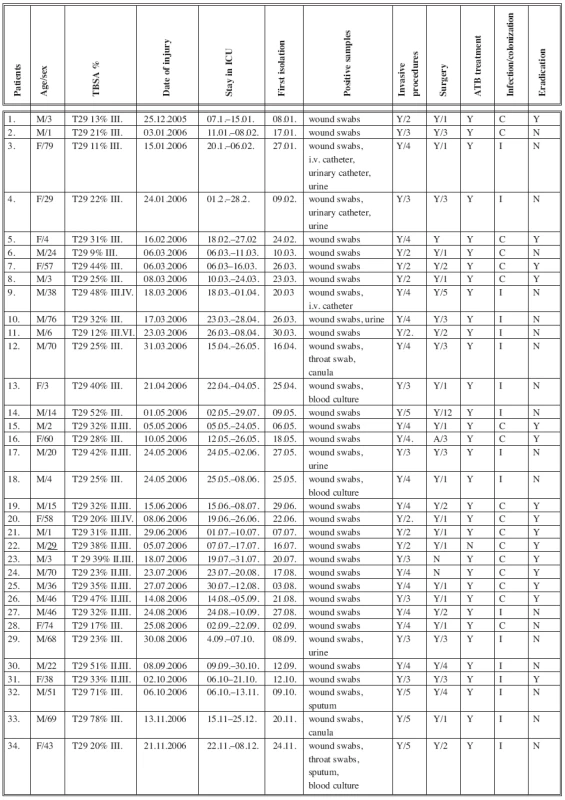
Table 3 shows that all patients with a positive isolation of A. baumannii strains fulfilled the criteria of the development of Acb colonization or infection confirmed by microbiological and laboratory tests. Sepsis in 3 patients, uroinfection in 5 patients, pneumonia in 2 patients and wound infection in 7 patients were also confirmed. Two patients (5.8%) died after multiorgan failure because of sepsis on 23rd and 29th post burn day. According to our observation, 24 (79%) A. baumannii isolates were in mixed cultures with other bacterial strains, such as Staphylococcus aureus, Pseudomonas aeruginosa, E. coli. etc.
Microbiological and clinical tests confirmed A. baumannii eradication in 15 patients (44%) which was performed by aggressive antimicrobial treatment.
Positive A. baumannii isolations in 19 (56%) patients persisted until all the wounds healed. Most successful eradication of wounds was performed by surgery and Sulfamylon solution. Sepsis and septic complications were treated by high doses of Meropenem, Imipenem/Cilastatin or a combination of Amikacin /Meropenem.
In 2006 Acinetobacter became the second bacterial strain most often isolated from the biological material of burn patients admitted to ICU (Fig. 2).
Obr. 2. Incidence of bacterial strains in ICU in 2006 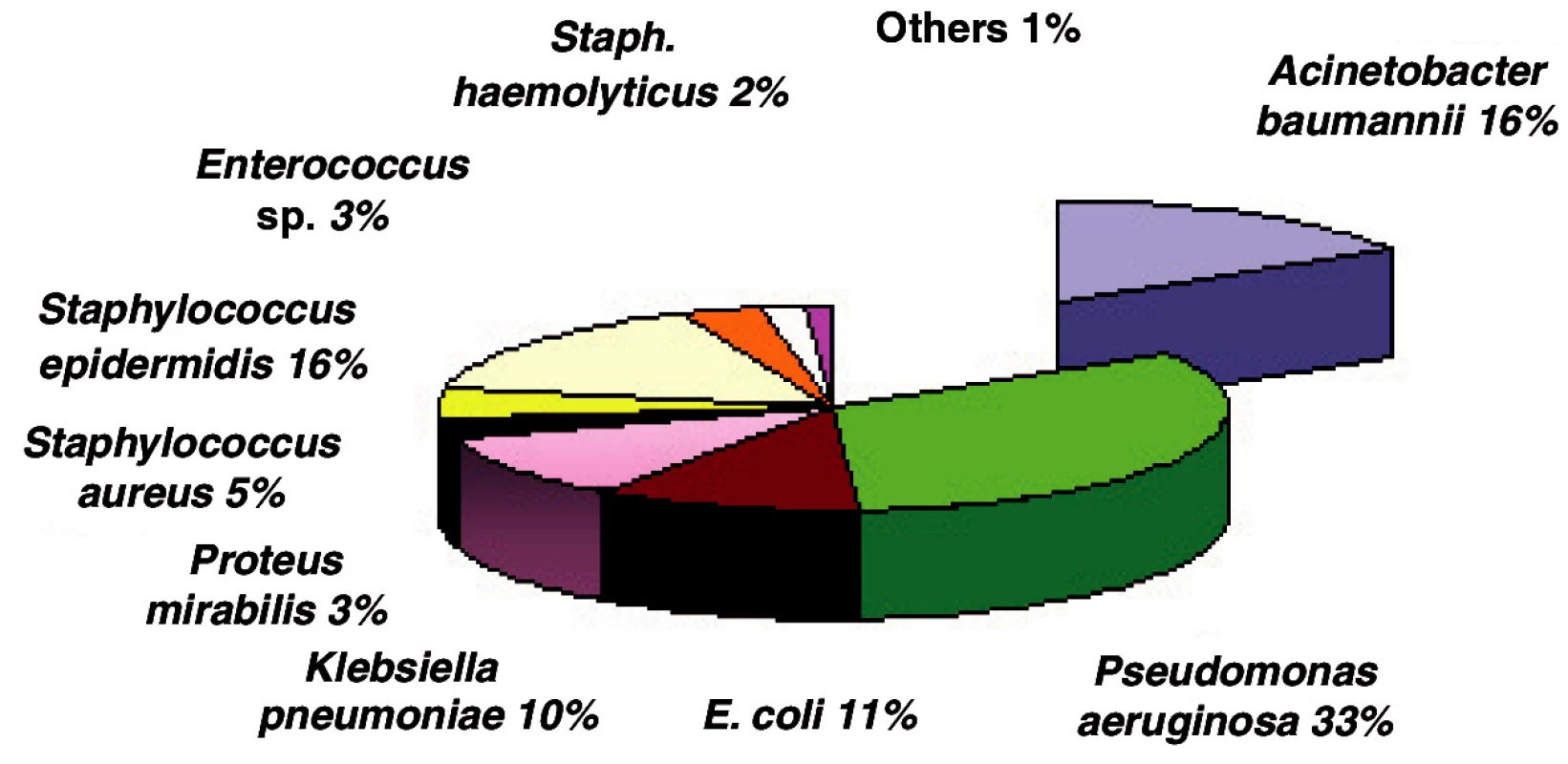
Tables 4-7 show the distribution of tested antimicrobials on isolated A. baumannii from Burn Department and ICU from 2003 to 2006.
Tab. 4. Distribution of Acinetobacter baumannii resistance (2003) 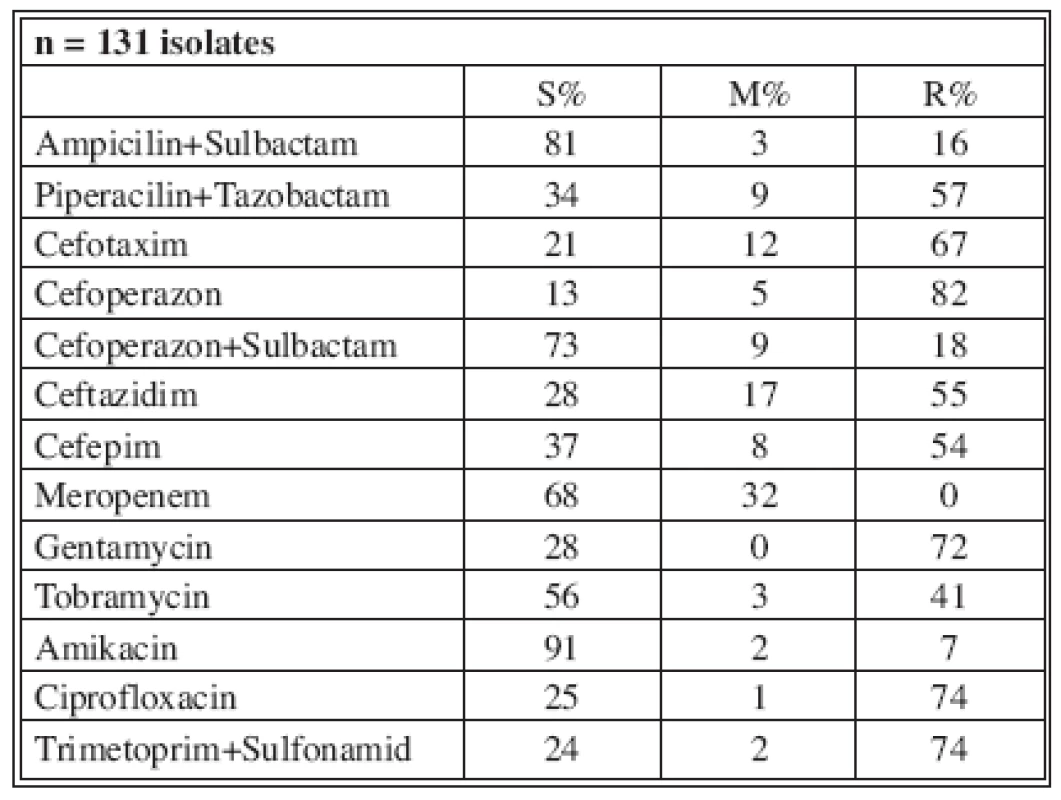
n* No. of isolates tested S* Percent susceptible M* Percent moderately susceptible R* Percent resistant Tab. 5. Distribution of Acinetobacter baumannii resistance (2004) 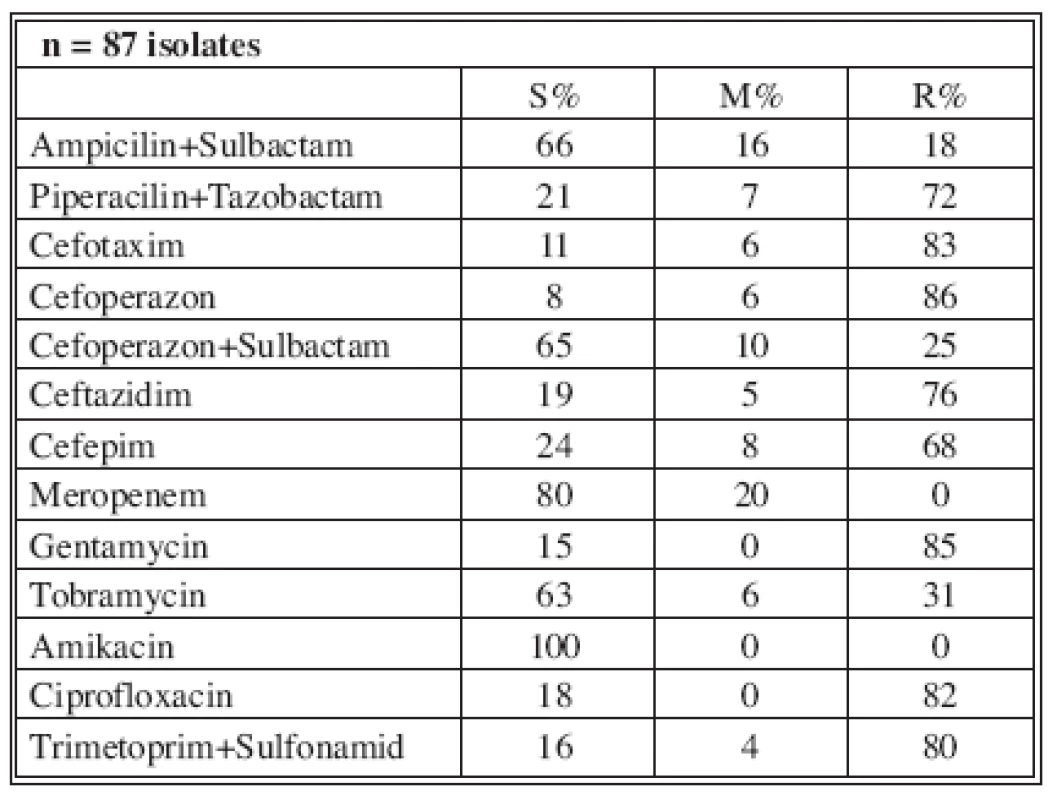
n* No. of isolates tested S* Percent susceptible M* Percent moderately susceptible R* Percent resistant Tab. 6. Distribution of Acinetobacter baumannii resistance (2005) 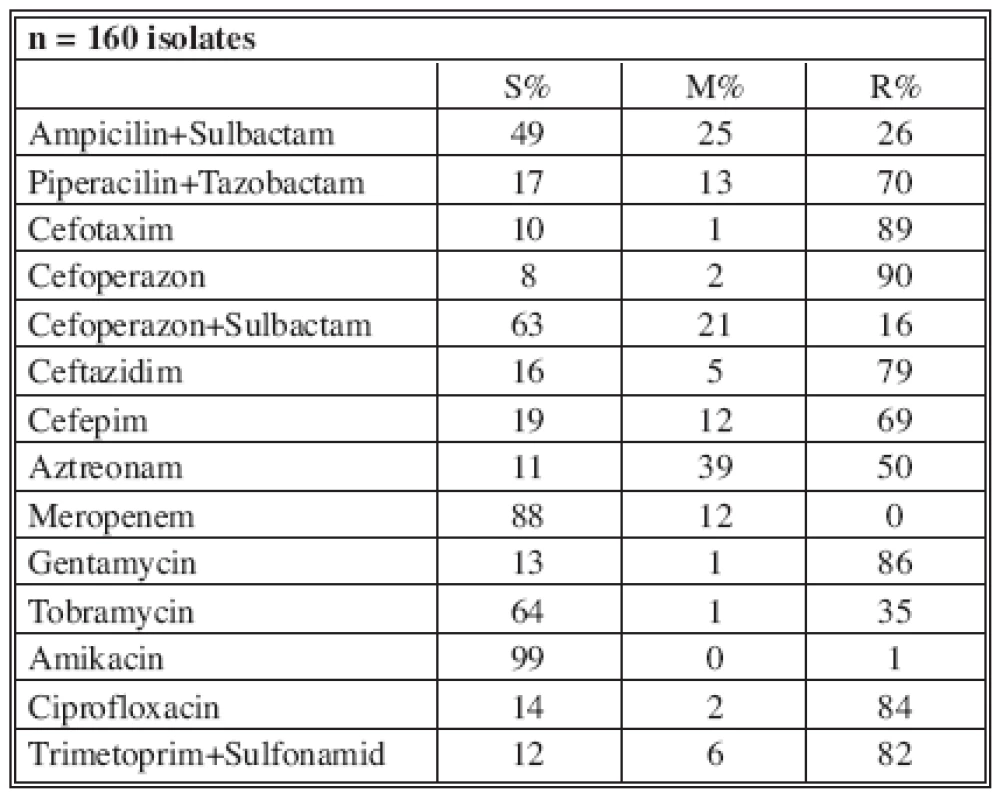
n* No. of isolates tested S* Percent susceptible M* Percent moderately susceptible R* Percent resistant Tab. 7. Distribution of Acinetobacter baumannii resistance (2006) 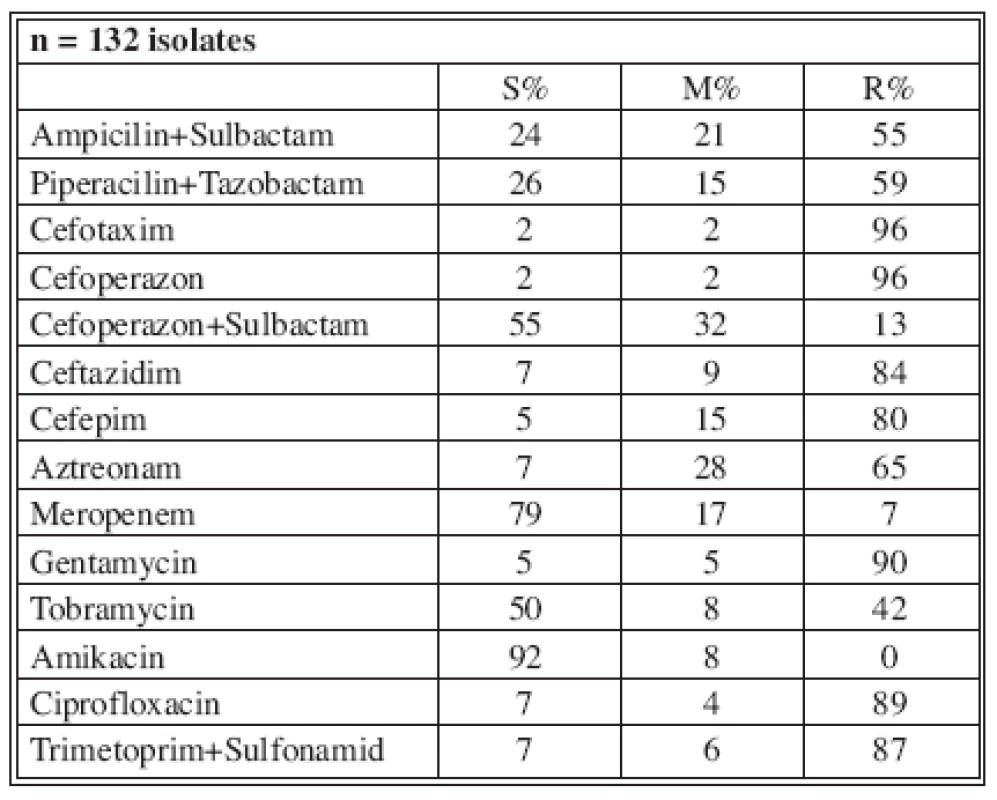
n* No. of isolates tested S* Percent susceptible M* Percent moderately susceptible R* Percent resistant Table 8 shows the resistance of tested antimicrobials on isolated A. baumannii from ICU in 2006. There were 6 (11%) multi-drug-resistant A. baumannii strains in ICU and one in burn department.
Tab. 8. Distribution of Acinetobacter sp. resistance in ICU (2006) 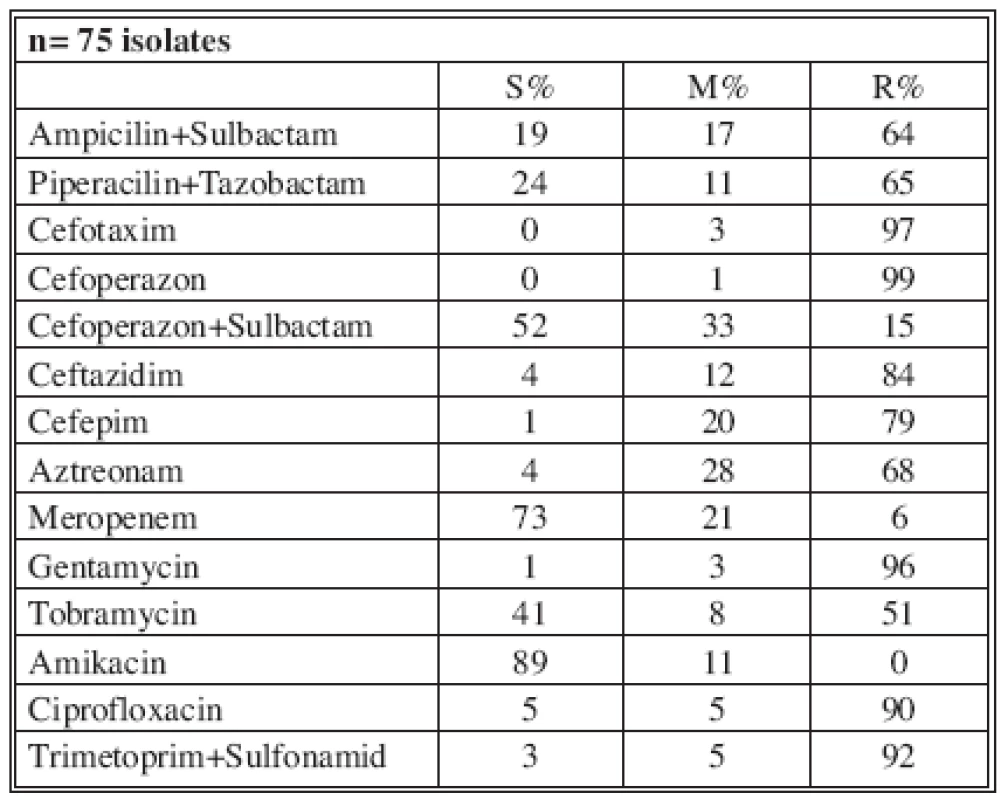
n* No. of isolates tested S* Percent susceptible M* Percent moderately susceptible R* Percent resistan Discussion
Fleming, Chain and Florey discovered penicillin and were awarded the Nobel Prize for Medicine in 1945. Gerhard Domagk was awarded the Nobel Prize in 1939 for his discovery of prontosil, the first sulfonamid (14). Their discovery and development revolutionized modern medicine and paved the way for the development of many more natural antibiotics. Antibiotics and Silversulfadiazin have dramatically changed burn treatment. Shortly after the introduction of potent and broad-spectrum antibiotics, the emergence of resistant strains became a major problem in ICUs (7). Despite advances in surgical care and new antimicrobial agents, infection and sepsis are responsible for over 50% mortality in burn patients worldwide (8). The organisms that predominate as causative agents of burn wound infection in any burn treatment unit change over time. Initially prevalent gram-positive infection is overgrown with gram-negative opportunists, mostly Pseudomonas aeruginosa and Acinetobacter baumannii (6, 9). The ability of Acinetobacter to survive in both very dry and wet conditions, their affinity for plastic and metal materials, and their unabrasive growth lead to failure in hygiene prophylaxis (12).
The increase in positive isolation of the Acb strains from the biological materials of burn patients, which was observed over recent years, and the very rapid development of resistance to antimicrobial medication, led us to the analysis of the problem. The ubiquitous microbe, with no clear clinical impact, has changed into a dangerous and very competitive nosocomial pathogen (28). This study also confirmed and sufficient data demonstrated serious danger for the burn patient from this pathogen.
In 2006 all patients included in this study with positive isolation of A. baumannii strains fulfilled the criteria of the development of A. baumannii colonization or infection by microbiological and laboratory testing. Two patients died after multi-organ failure because of Acinetobacter sepsis.
Identifying infections in the ICU can be challenging. Bedside imaging and laboratory markers such as neutrophil count, erythrocyte sedimentation rate and C reactive protein (CRP) are nonspecific and cannot reliably confirm bacterial infection. Procalcitonin (PTC) may be a useful prognostic marker to indicate bacterial infection. A positive blood test and quantitative bacterial culture of resistant A. baumannii represent a high risk for patient, and because of the limited number of effective ATB this is a challenge for everyone who cares for burn patients (25). Carbapenems are limited; only Colimycin, Riphampicin and the new ATB like Tigecyclin may help to solve septic complications.
According to our observation 24 (79%) of A. baumannii isolates were present in mixed cultures with other bacterial strains such as Staphylococcus aureus, Pseudomonas aeruginosa, E. coli, Proteus sp.
The eradication of A. baumannii strains was successful in 15 (44%) patients, which was confirmed clinically and microbiologically. In 19 patients (56%), positive A. baumannii isolations persisted during the treatment.
Conclusion
There is a high risk to receive Acinetobacter infection during the hospital stay. On average 79% of patients had their first positive isolation of A. baumannii strains on the sixth day of hospitalisation in the ICU. Only 21% patients had their positive isolation within 48 hours of admission. All patients with a positive isolation of A. baumannii fulfilled all the criteria of the development of A. baumannii colonization or infection (see Table 3). Clinical, microbiological and other laboratory examination confirmed that 17 (50%) patients developed A. baumannii infection. There was sepsis in 3 patients, pneumonia in 2 patients, uroinfection in 5 patients and wound infection in 12 patients. Wounds and mucosa colonization was confirmed in 50% of patients.
Microbiologically and clinically confirmed eradication of A. baumannii strains from wounds was successful in 15 patients. In 19 patients (56%), positive A. baumannii isolations persisted until all the wounds healed.
In general, first, second and third generation cephalosporins, macrolides, and penicillins have little or no anti-Acinetobacter activity, and their use may predispose to Acinetobacter colonization (6).
As summarized by Go and Cunha (1999), medication to which Acinetobacter is usually sensitive include Meropenem, Colistin, Amikacin, Minocycline and Tigecycline (6). Colonization of one patient may result in infection in another patient. For these reasons, every attempt should be made to isolate patients who are colonized with Acinetobacter in order to prevent other patients from becoming colonized (26). According to our observation 24 (79%) of A. baumannii isolates were present in mixed cultures with other bacterial strains such as Staphylococcus aureus, Pseudomonas aeruginosa, Enterobacter spp. In our opinion there are several possibilities to minimize the problem of A. baumannii infections or colonizations of the burned patient.
- Make aĘhigh hygienic standard in ICU
- Continued surveillance of prevalent microorganisms in ICU
- Providing strict isolation of the colonized or infected patients
- Minimize diagnostic or therapeutic interventions
- Reduce selection pressure, including more frequent rotation of antibiotic groups
- Improve macrobiotic techniques to allow earlier and more accurate identification
- New issues in hospital epidemiology include the standardisation and quality control of methods, automated data acquisition
Address for correspondence:
Jan Babík, M.D.
Clinic for Burns and Reconstructive Surgery
Košice-Šaca
Lúčna 57
040 15 Košice-Šaca
Slovakia
E-mail: jbabik@nemocnicasaca.sk
Zdroje
1. Altoparlak U., Erol S., Akcay MN. The time-related changes of antimicrobial resistance patterns and predominant bacterial profiles of burn wounds and body flora of burned patients. Burns, 30, 2004, p. 660-664.
2. Baumann P., Isolation of Acinetobacter from soil and water. J. Bact., 96, 1968, p. 39-42.
3. Rusin P., Rose J., Haas C., Gerba C. Risk assessment of opportunistic bacterial pathogens in drinking water. Rev-Environ-Toxicol., 152, 1997, p. 57-83.
4. Gibran NS., Heimbach, DM. Current status of burn wound pathophysiology. Clin. Plast. Surg., 2, 2000, p. 11-22.
5. Corbella X., Pujol, M., Ayats J., et al. Relevance of digestive tract colonization in epidemiology of nosocomial infections due to multiresistant Acinetobacter baumannii. Clinical Infectious Diseases, 23, 1996, p. 329-334.
6. Cunha BA., Infections caused by non-fermentative aerobic gram-negative bacilli in the critical care unit., Antibiotics for Clinicians, 4, 2000, p. 11-16.
7. Pruitt BA., McManus AT., Kim SH. Burn wound infections: current status. World J. Surg., 22, 1998, p. 35-45.
8. McManus AT., Kim SH., McManus WF., et al. Comparison of quantitative microbiology and histopathology in divided burn-wound biopsy specimens. Arch. Surg., 122, 1987, p. 74-76.
9. Bouvet PJM., Grimont PAD. Taxonomy of the genus Acinetobacter with the recognition of Acinetobacter baumannii sp. nov., Acinetobacter haemolyticus sp. nov., Acinetobacter johnsonnii sp. nov., and Acinetobacter junii sp. nov., and amended description of Acinetobacter calcoaceticus and Acinetobacter Lwofii. Int. J. Syst. Bact., 36, 1986, p. 228-240.
10. Nemec A., Taxonomie rodu Acinetobacter. Epidemiol. Imunol., 45, 1996, p. 23-29.
11. Weernink A., Severin WPJ., Tjernberg I., Dijkshoorn, L. Pillows, an unexpected source of Acinetobacter. J. Hosp. Infect., 29, 1995, p. 189-199.
12. Sheretz RJ., Sulivan,ĘML. An outbreak of infections with Acinetobacter calcoaceticus in burn patients: contamination of patients mattresses. J. Infect. Dis., 151, 1985, p. 252-258.
13. Honari S.ĘTopical therapies and antimicrobials in the management of burn wounds. Crit. Care Nurs. Clin. North Am., 16, 2004, p. 1-11.
14. Domagk, G. The Columbia Encyclopedia, 6th Ed., Copyright 2007, Columbia University Press, 2007, p. 155.
15. Anstey NM., Currie JB., WithnallĘKM. Community-acquired Acinetobacter pneumonia in the Northern Territory of Australia. Clin. Infect. Dis., 14, 1992, p. 83-91.
16. Sule O., Ludlam HA., Walker CW., Brown DF., Kaufmann ME. AĘpseudo-outbreak of respiratory infection with Acinetobacter sp. Infect. Control Hosp. Epidemiol., 18, 1997, p.Ę510Đ512.
17. Mulin B., Rouqet C., Clement C., Bailly P., Julliot MC., Viel JF., Thouverez M., Vielle I., Barale F., Talon D. Association of private isolation rooms with ventilator-associated Acinetobacter baumannii pneumonia in surgical intensive-care unit. Infect. Control Hosp. Epidemiol., 18, 1997, p. 499-503.
18. Fagon JY., Chastre J., Domart Y., Rouillet JL., Gilbert C. Mortality due to ventilator-associated pneumonia or colonization with Pseudomonas or Acinetobacter species: Assessment by quantitative culture of samples obtained by aĘprotected specimen brush. Clin. Infect. Dis., 23, 1996, p.Ę538Đ542.
19. Seifert H., Strate A., PulvererĘG. Nosocomial bacteremia due to Acinetobacter baumannii. Clinical features, epidemiology, and predictors of mortality. Medicine (Baltimore), 74, 1995, p. 340-349.
20. Cisneros JM., Reyes MJ., Pachon J., Becerril B., Caballero FJ., Garcia-Garmendia JL., Ortiz C., Cobacho AR. Bacteremia due to Acinetobacter baumannii: epidemiology, clinical findings, and prognostic features. Clin. Infect. Dis., 22, 1996, p. 1026-1032.
21. Baraibar J., Correa H., Mariscal D., Gallego M., Valles J., Rel - lo J. Risk factor for infection by Acinetobacter baumannii in intubated patients with nosocomial pneumonia. Chest, 112, 1997, p. 1050-1054.
22. Poutanen SM., Louie M., Simor AE. Risk factor, clinical features and outcome of Acinetobacter bacteremia in adults. Eur. J. Clin. Microbiol. Infect. Dis., 16, 1997, p. 737-740.
23. Gospodarek E., Sieradzka E., Bugalski R. Presence of various Acinetobacter species in urinary tract infections. Med. Dosw. Mikrobiol., 43, 1991, p. 111-118.
24. Lesseva MI., Hadjiski OG. Analysis of bacteriuria in patients with burns. Burns, 21, 1995, p. 3-6.
25. Cardany CR., Rodeheaver GT., Horowitz JH., Kenney JG., Edlich RF. Influence of hydrotherapy and antiseptic agents on burn wound bacterial contamination. J. Burn Care Rehabil., 6, 1985, p. 230-232.
26. McManus AT., Mason AD., McManus WF. A decade of reduced gram-negative infections and mortality associated with improved isolation of burned patients. Arch. Surg., 129, 1994, p. 1306-1309.
27. Seifert H., Dijkshoorn L., Gerner-Smidt P., Pelyer N., Tjernberg-Vaneechoutte M. Distribution of Acinetobacter species on human skin: comparison of phenotypic and genotypic identification metods. J. Clin. Microbiol., 35, 1997, p. 2819-2825.
28. Koeleman JG., Parlevliet GA., Dijkshoorn, L., Savelkoul PH., Vandebroucke-Grauls, CM. Nosocomial outbreak of multi-resistant Acinetobacter baumannii on a surgical ward: epidemiology and risk factors for acquisition. J. Hosp. Infect., 37, 1997, p. 113-123.
Štítky
Chirurgia plastická Ortopédia Popáleninová medicína Traumatológia
Článek ČESKÉ A SLOVENSKÉ SOUHRNYČlánek HOW EAST DISCOVERED WEST
Článok vyšiel v časopiseActa chirurgiae plasticae
Najčítanejšie tento týždeň
2008 Číslo 1- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Kombinace paracetamolu s kodeinem snižuje pooperační bolest i potřebu záchranné medikace
- Metamizol v kostce a v praxi – účinné neopioidní analgetikum pro celé věkové spektrum
- Kombinace tramadol/paracetamol zmírňuje bolest v oblasti bederní páteře a může tak zmírnit i depresi
-
Všetky články tohto čísla
- MutilATing electrotrauma – case REPORT
- Award of the G. Whitaker International Burns Prize for 2007
- Specific aspects of the treatment of patients with multiple mechanical and burn injuries
- Catheter-Related Infections in Burn Patients at the Burn Centre of the University Hospital in Ostrava
- G. WHITAKER INTERNATIONAL BURNS PRIZE – PALERMO (Italy)
- Acinetobacter – serious danger FOR burn patients
- THE 25TH ANNIVERSARY OF BRNO BURN CENTRE: WHAT HAS CHANGED AND WHAT HAS NOT
- THE EFFECT OF NIFEDIPINE ON THE PATENCY OF MICROVASCULAR ANASTOMOSIS IN RATS
- Report of the 12th Congress of European Burns Association (EBA), Budapest, Hungary, September 12–15, 2007
- ČESKÉ A SLOVENSKÉ SOUHRNY
- HOW EAST DISCOVERED WEST
- Acta chirurgiae plasticae
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Specific aspects of the treatment of patients with multiple mechanical and burn injuries
- Acinetobacter – serious danger FOR burn patients
- THE EFFECT OF NIFEDIPINE ON THE PATENCY OF MICROVASCULAR ANASTOMOSIS IN RATS
- MutilATing electrotrauma – case REPORT
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



