-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Routine Pediatric Enterovirus 71 Vaccination in China: a Cost-Effectiveness Analysis
Using surveillance and survey data, Joseph T. Wu and colleagues assess the cost-effectiveness of routine pediatric EV71 vaccination in China.
Published in the journal: Routine Pediatric Enterovirus 71 Vaccination in China: a Cost-Effectiveness Analysis. PLoS Med 13(3): e32767. doi:10.1371/journal.pmed.1001975
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001975Summary
Using surveillance and survey data, Joseph T. Wu and colleagues assess the cost-effectiveness of routine pediatric EV71 vaccination in China.
Introduction
Since the 1990s, large epidemics of hand, foot, and mouth disease (HFMD) have occurred across the Western Pacific region [1–6]. In China, which accounted for 87% (9.8 million/11.3 million) of all HFMD cases reported to WHO during 2010–2014 [7], HFMD epidemics have been occurring annually since 2007. Between 2008 and 2013, China’s national HFMD surveillance registered around 9 million cases and 2,700 deaths, 90% and 96% of which, respectively, occurred in children under 5 y [6]. In 2012, HFMD ranked first among all notifiable diseases in China by both case count and deaths for children under 5 y [8].
Enterovirus 71 (EV71) is a major causative pathogen of HFMD epidemics. In China, EV71 accounted for more than 90% of laboratory-confirmed fatal HFMD cases between 2008 and 2013 [6]. EV71-associated HFMD (EV71-HFMD) causes substantial morbidity and mortality because effective therapeutic and preventive measures remain elusive. There is no established antiviral treatment for EV71-HFMD [9]. The prevention measures suggested by WHO are hand hygiene and social distancing, the efficacy of which are uncertain [10,11].
Three inactivated monovalent EV71 vaccines (each requiring two doses administered 4 wk apart) manufactured in China were recently shown in phase III trials to be safe and efficacious against EV71-HFMD for children aged 6–71 mo [12–14]. Economic evaluation is important in considering population rollout of any new vaccine [15]. Here we provide such an evaluation using the best available data comprising (i) national HFMD surveillance, (ii) HFMD virological surveillance records from all 31 provinces, and (iii) a nationwide caregiver survey on the economic costs and health utility loss associated with EV71-HFMD.
Methods
Ethics Statement
Ethical approval (approval number 201417) was acquired from the institutional review board of the Chinese Center for Disease Control and Prevention. Caregiver survey interviews were conducted only after verbal informed consent was obtained from a parent or caregiver of the pediatric patient. In May 2008, HFMD was added to the list of notifiable diseases in China. According to China’s law on the prevention and treatment of infectious diseases, personal identifiers should be collected for individual cases with diagnosis of a notifiable disease, for the purposes of public health surveillance and response. The National Health and Family Planning Commission of China decided that the collection of individual data for all notifiable diseases, including HFMD, according to the national surveillance protocol was part of an ongoing public health response and was thus exempt from institutional review board assessment.
Model
We used a static model to perform a cost-effectiveness analysis (CEA) of routine pediatric EV71 vaccination in China (i.e., a model with no indirect protection). In a separate study, we used a time series susceptible–infected–recovered (TSIR) dynamic model to show that transmissibility of EV71-HFMD in China is high (with a national average basic reproductive number of around 27) and that indirect protection from EV71 vaccination is unlikely to be significant [16]. Herd immunity appears only at very high vaccine coverage levels and hence can be ignored at the coverage levels considered in our analysis. As such, we have chosen to use a static model for the CEA because it provides a simple yet very accurate model of the reduction in EV71-HFMD incidence conferred by routine pediatric EV71 vaccination. S1 Fig shows that the long-term incidence reduction predicted by the static model is essentially the same as that predicted by the TSIR dynamic model. See “Indirect Protection Conferred by Vaccination” in S1 Text for a more detailed explanation of this phenomenon.
Fig 1 summarizes the model structure and parameterization, with further details provided in “The Model” in S1 Text. We considered only children aged 6 mo to 5 y (60 mo) because (i) 90%, 94%, and 96% of mild, severe, and fatal HFMD cases, respectively, occur in this age group [6] and (ii) vaccine efficacy has been demonstrated only in those aged 6–71 mo [12–14]. The life expectancy of each birth cohort was assumed to be 75 y [17].
Fig. 1. Model structure and parameterization. 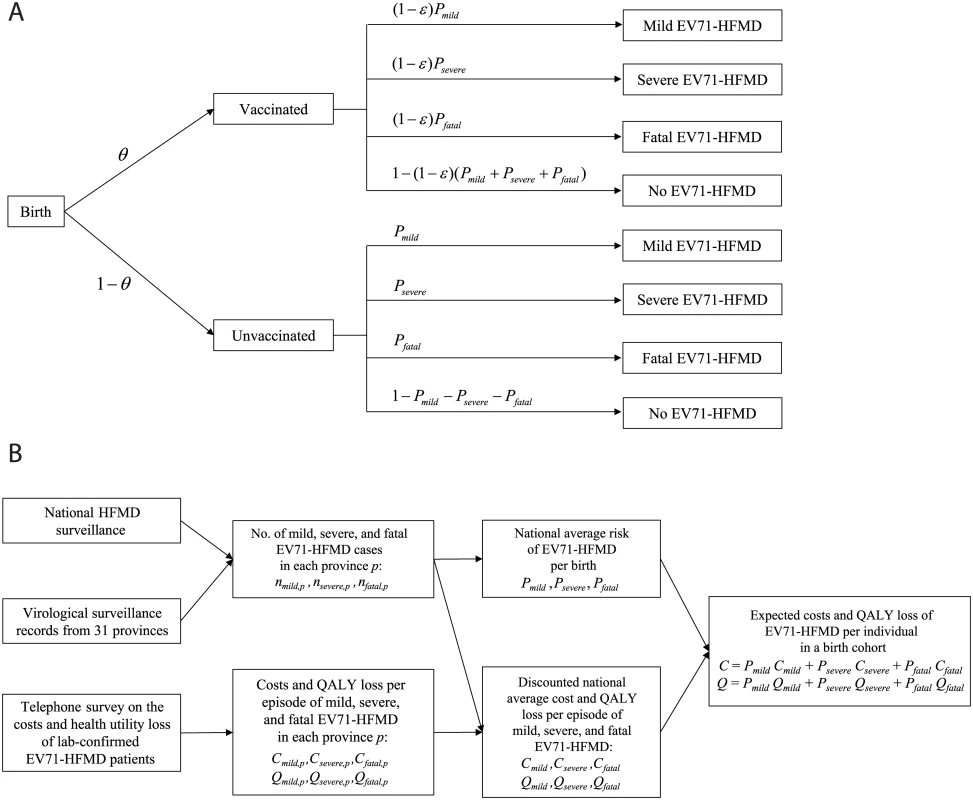
(A) The static model used in the CEA with vaccine coverage θ and vaccine efficacy ε. (B) A schematic that describes how the data sources were used to parameterize the model. See S1 Text for more details. QALY, quality-adjusted life year. We assumed that (i) children would be vaccinated 6 mo after birth, with immediate and constant efficacy ε against EV71-HFMD for at least 5 y, and (ii) conservatively, if vaccinated children developed EV71-HFMD, the probability of severe outcomes was the same as that for unvaccinated children. Consequently, the risk of EV71-HFMD in vaccinated children was reduced by a proportion ε for all levels of severity.
Data
We parameterized the model using the following data.
National HFMD surveillance dataset during 2010–2013 (S1 Data)
China’s national HFMD surveillance system has been described in our previous report [6]. Briefly, the system classifies HFMD cases as severe if the patient experiences neurological, respiratory, or cardiopulmonary complications; otherwise, cases are classified as mild [6]. Virological surveillance requires all hospitals to collect specimens for serotyping from (i) all severe and fatal cases and (ii) the first five mild cases every month. Serotyping results were classified into four categories: EV71, Coxsackievirus A16 (CA16), other enterovirus (OEV), or test-negative for enteroviruses. The system, however, does not record test-negative results. While some test-negative cases could be misdiagnoses (e.g., chicken pox or measles), others could be bona fide EV71-HFMD cases but with negative test results because of late collection of specimens, poor specimen quality, or other reasons. To account for the uncertainty regarding the percentage of test-negative cases that were EV71-HFMD, we acquired internal laboratory records from all 31 provinces (below) to supplement our analysis.
Virological surveillance records from all 31 provinces (S1 Data)
This dataset contained the weekly number of EV71, CA16, OEV, and test-negative results in each province from 1 January 2010 to 31 December 2013 (Fig 2A). These records contained no severity information until 31 December 2012. As such, we considered 19 test-negative scenarios (scenarios A–S in Fig 2B) that corresponded to assumptions regarding (i) the percentage of test-negative cases that were mild, (ii) the percentage of test-negative mild cases that were EV71-HFMD, and (iii) the percentage of test-negative severe/fatal cases that were EV71-HFMD. As shown in Fig 2B, there were 21 original scenarios, but three of them were identical, hence only 19 unique scenarios. See “Uncertainty in Test-Negative Cases” in S1 Text for details.
Fig. 2. Estimating the percentage of mild and severe HFMD cases attributed to EV71 in China. 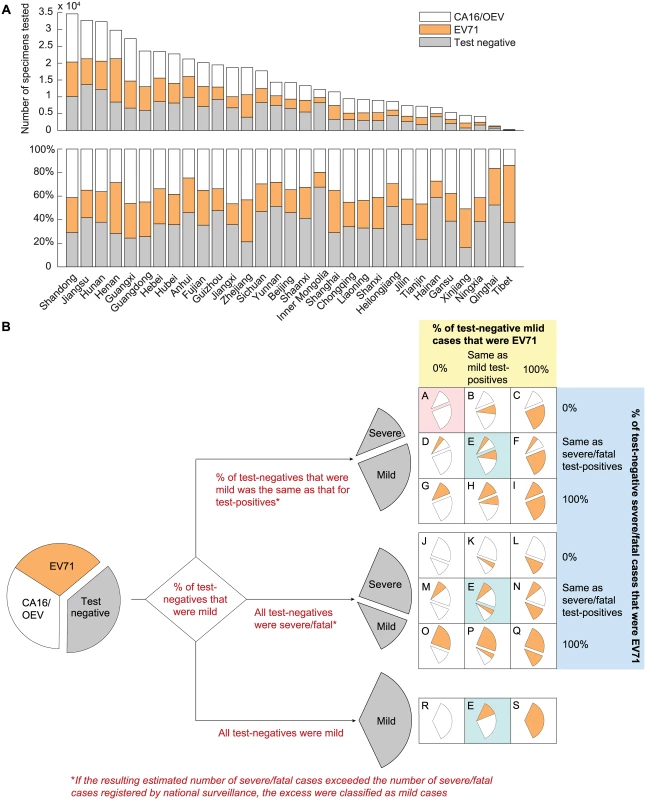
(A) The number of specimens tested and the distribution of test results in each of the 31 provinces during 2010–2013. (B) Twenty-one scenarios were generated by making assumptions regarding the percentage of test-negative cases that were mild during 2010–2012 (the first branching point; annotated in red text) and the percentage of test-negative mild and severe/fatal cases that were EV71 during 2010–2013 (the second branching point; annotated in yellow and blue shades), respectively. The outcomes in the three scenarios colored in cyan were identical; hence, there were only 19 unique scenarios (labeled A–S). The base case (scenario A) is colored in pink. The sizes of the wedges illustrate the underlying assumptions and are not proportional to the actual percentages (the differences in serotype and severity distribution for severe/fatal cases between scenarios would not be apparent otherwise because the number of mild cases was much larger than that of severe/fatal cases). Caregiver survey
A telephone survey was conducted of parents or caregivers of children aged 6 mo to 5 y with laboratory-confirmed HFMD diagnosed between 1 January 2012 and 17 December 2013 as registered by national surveillance (S2 and S3 Data). Information about the child’s diagnosis and treatment and household expenses, time off work, and quality of life related to the HFMD episode was obtained from respondents who consented to be interviewed. Health-related quality of life was measured using the EuroQol EQ-5D-3L instrument (http://www.euroqol.org/). Briefly, we contacted 29,810 parents or caregivers of HFMD patients, and 3,500 agreed to participate in our survey. Of these, 1,787 were parents or caregivers of EV71-HFMD patients. The mean total cost of a pediatric EV71-HFMD episode reported in China was US$233, US$1,173, US$3,279, and US$2,738 for mild outpatient, mild inpatient, severe, and fatal cases, respectively. The mean quality-adjusted life year (QALY) loss per episode was 0.0039, 0.0070, 0.0194, and 30.4, respectively. We found little evidence of associations between costs or QALY loss and patients’ age, sex, or urban residence, so we stratified costs and QALY loss by case severity and geographical region only. We estimated the mean cost and QALY loss per episode using the central limit theorem and accounted for the associated parametric uncertainty in probabilistic sensitivity analysis. See “Survey on Household Costs and Quality of Life Detriment Associated with EV71-HFMD” in S1 Text for details.
Burden of Disease
We assumed that without vaccination, the risk of EV71-HFMD at all levels of severity for future birth cohorts (denoted by Pmild, Psevere, and Pfatal) would be similar to that registered by national surveillance during 2010–2013. At each level of severity s, Ps comprised the risk of EV71-HFMD for the corresponding level of severity in each province (Fig 1). See “The Model” in S1 Text for mathematical details.
Costs and Health Utility Loss Due to EV71-HFMD
The cost of EV71-HFMD per birth, C, was estimated as the weighted sum of the average cost of mild, severe, and fatal cases (Cmild, Csevere, and Cfatal), where the weights were simply the risk of mild, severe, and fatal EV71-HFMD (Pmild, Psevere, and Pfatal). The health utility loss attributable to EV71-HFMD, Q, was estimated analogously from the health utility loss of mild, severe, and fatal cases (Qmild, Qsevere, and Qfatal). Cost and health utility loss at each level of severity were discounted according to the age distribution of cases for the corresponding level of severity in each province. We did not account for adverse events following immunization because such events in the phase III trials were largely mild and uncommon [12–14]. We excluded productivity loss due to pediatric premature death because of unresolved debate about the way such losses should be costed [18]. See “The Model” in S1 Text for details.
Cost-Effectiveness
The incremental cost-effectiveness ratio (ICER) of vaccine introduction was calculated as follows:
where vaccine cost was the cost per child fully vaccinated, which included the procurement, logistical, and administration cost for the necessary number of vaccine doses to confer 5 y of protection. Because the three EV71 vaccines have significantly different efficacy estimates [12–14] and reliable estimates of their costs are not yet available, we used effective vaccine cost (EVC), defined as (vaccine cost)/(vaccine efficacy), which is simply the cost adjusted for efficacy, or the cost of a 100% efficacious vaccine, as the outcome for our CEA. Given a willingness-to-pay threshold, routine pediatric EV71 vaccination would be cost-effective if and only ifWe denote this ceiling by EVCmax hereafter. With EVCmax as the outcome of our CEA, we can easily obtain the cost-effective vaccine cost ceiling of any given vaccine by multiplying EVCmax by the corresponding vaccine efficacy.
Base Case
We performed the CEA from the societal perspective [19]. Costs and health utility loss were discounted at 3% per annum [20]. We used one times the gross domestic product per capita (GDPpc) (US$6,700 in 2013 for China [21]), which is the WHO-CHOICE criterion for very cost-effective interventions [20], as the societal willingness-to-pay threshold. In terms of assumptions regarding test-negative cases, we used the most conservative assumptions in the base case (colored in pink in Fig 2B) in order to avoid overestimating the cost-effectiveness of vaccination. This corresponded to assuming that (i) the percentage of test-negative cases that were mild was the same as that for test-positive cases (at the first branching point in Fig 2B) and (ii) none of the test-negative cases were EV71 (at the second branching point in Fig 2B).
Uncertainty Analysis
We considered all possible combinations of the following decision-making uncertainty associated with the choices of CEA parameters: (i) inclusion or exclusion of productivity loss of parents or caregivers due to caring for their sick children (i.e., indirect non-medical costs), (ii) discounting costs and health utility loss at 3% or 6%, and (iii) using one or three times GDPpc as the willingness-to-pay threshold (the latter is the WHO-CHOICE criterion for cost-effective interventions [20]). For each combination of these possibilities (no probability distributions are imposed on these parameters), we calculated EVCmax for all 19 test-negative scenarios in Fig 2B.
In addition, we considered the uncertainty associated with under-ascertainment and underreporting [22]. The validity of our CEA substantially depends on the proportion of EV71-HFMD cases not registered by national surveillance. To estimate this proportion, we compared the incidence rate of EV71-HFMD reported in the EV71 vaccine phase III trials (in which intensive active surveillance for HFMD was conducted in one county of Beijing, six counties of Jiangsu, and seven counties of Guangxi) with the incidence rate of EV71-HFMD registered by national surveillance in the counties and time periods of these trials [12–14]. We estimated the EV71-HFMD incidence rate in each trial using the number of participants and EV71-associated diseases in the placebo group. See “Estimation of the Unregistered Proportion” in S1 Text for details. We then repeated the calculation of EVCmax in all of the above-mentioned scenarios assuming that the resulting unregistered proportion was generalizable to the whole country and that the expected cost and QALY loss of registered and unregistered cases were similar.
Probabilistic Sensitivity Analysis
In each scenario, we performed probabilistic sensitivity analysis to account for parametric uncertainty in our estimates of the risk of EV71-HFMD (Pmild, Psevere, Pfatal) and the expected costs and QALY loss per case due to EV71-HFMD (Cmild, Csevere, Cfatal, Qmild,, Qsevere, Qfatal; see “Survey on Household Costs and Quality of Life Detriment Associated with EV71-HFMD” in S1 Text for details). When considering the unregistered proportion in the uncertainty analysis, we assumed the proportion of EV71-HFMD cases registered by national surveillance followed a beta distribution that corresponded to the mean and 95% CI obtained from the comparison of incidence rate between the EV71 vaccine phase III trials and the national surveillance database. See Table 1 for details on the probability distributions used in the probabilistic sensitivity analysis.
Tab. 1. Parameters for the cost-effectiveness model. 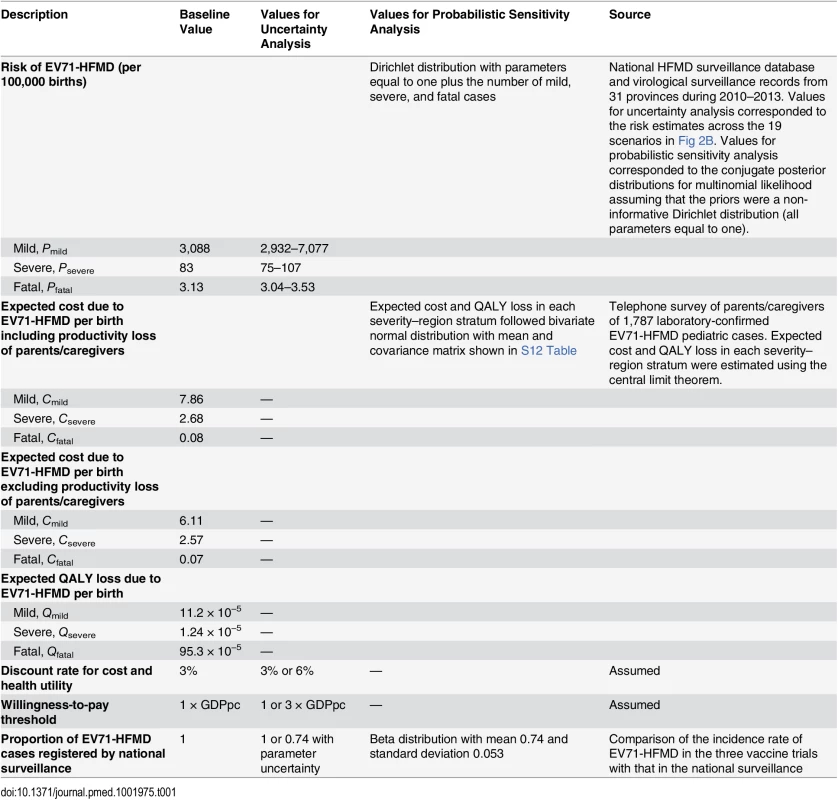
Comparative Cost-Effectiveness Analysis of the Three Vaccines
The three EV71 vaccines have different efficacy estimates, and reliable estimates of their costs are not yet available. As such, we used EVCmax to identify cost regions where each vaccine would be preferred given uncertainty in their efficacies. Specifically, because EVC is simply the cost per unit of vaccine efficacy purchased, it could be used to compare the incremental cost-effectiveness of any two vaccines as follows (no vaccination is just a special case with US$0 cost and no efficacy). If vaccines A and B have efficacies εA > εB and their costs are VA > VB, choosing vaccine A over vaccine B would be cost-effective if and only if (VA − VB)/(εA − εB) < EVCmax. For each possible combination of costs for the three vaccine candidates, we used this criterion to identify the candidate that has the highest probability of being the optimal vaccine by assuming that 1 − vaccine efficacy for the three vaccines followed log-normal distributions such that the medians and 2.5th and 97.5th percentiles of the associated vaccine efficacies were the same as the point estimates and 95% CIs of the vaccine efficacies reported in the clinical trials [12–14]. Please see “Comparative CEA of the Three Vaccine Candidates” in S1 Text for more details.
Results
Base Case
In the base case, the risk that a child would develop mild, severe, and fatal EV71-HFMD between age 6 mo and 5 y was 3,088 (95% CI 3,084–3,093), 83.4 (95% CI 82.6–84.2), and 3.1 (95% CI 3.0–3.3) per 100,000, respectively (Fig 3A; Table 1). The estimated cost per birth attributed to EV71-HFMD was US$10.6 (95% CI US$9.7–US$11.5); 74%, 25%, and 1% of this cost was incurred by mild, severe, and fatal cases, respectively (Fig 3B). Pediatric EV71 vaccination would be cost-saving if EVC was below this amount.
Fig. 3. The risk, expected costs, and health utility loss of EV71-HFMD per birth in the base case. 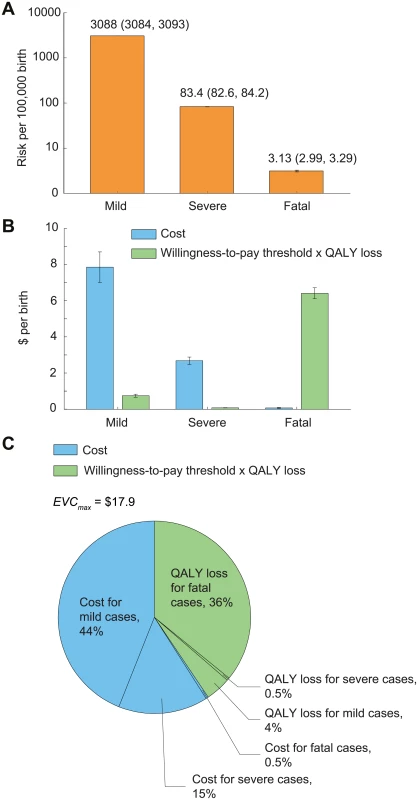
Error bars indicate 95% CIs. (A) Risk of mild, severe, and fatal EV71-HFMD. The 95% CIs are annotated because they are not graphically apparent. (B) Expected costs and health utility loss attributable to EV71-HFMD stratified by severity. To indicate the relative contribution of cost and health utility loss in EVCmax, health utility loss was expressed in monetary terms as the product of QALY loss and willingness-to-pay threshold, which is set at one times GDPpc (US,700). (C) Percentage breakdown of EVCmax. The expected QALY loss attributed to EV71-HFMD per 10,000 births was 10.8 (10.7–10.9); 89%, 10%, and 1% of this loss was incurred by fatal (almost entirely due to premature death), mild, and severe cases, respectively (Fig 3B). With a willingness-to-pay threshold of one times GDPpc, pediatric EV71 vaccination was cost-effective if EVC was below US$17.9 (US$16.9–US$18.8). The reduction in costs and averted QALY loss (in monetary terms) constituted 60% and 40% of the net monetary benefit of vaccination, respectively (Fig 3C).
Uncertainty Scenarios
Mild cases were a major constituent of EVCmax (Fig 3C), and the risk of mild EV71-HFMD, and hence EVCmax, strongly depended on the percentage of test-negative mild cases that were EV71 (Table 2; Fig 4A and 4B). In contrast, EVCmax was relatively insensitive to the percentage of test-negative severe/fatal cases that were EV71 because (i) severe cases were only a minor constituent of EVCmax (Fig 3C) and the risk of severe EV71-HFMD varied less across scenarios (Fig 4C), and (ii) although fatal cases were a major constituent of EVCmax (Fig 3C), the risk of fatal EV71-HFMD was almost constant across scenarios (Fig 4D). Compared to EVCmax in the base case (which was the lowest and hence the most conservative among all scenarios), EVCmax increased by (i) 19%–32% if the percentage of test-negative mild cases that were EV71 was the same as that for test-positive mild cases (scenarios K, B, E, H, and P in Tables 2 and S5 and Fig 4A) and (ii) 50%–67% if all test-negative mild cases were EV71 (scenarios L, C, N, Q, F, I, and S in Tables 2 and S5 and Fig 4A).
Fig. 4. Cost-effectiveness of routine pediatric EV71 vaccination in China with a willingness-to-pay threshold of one times GDPpc. 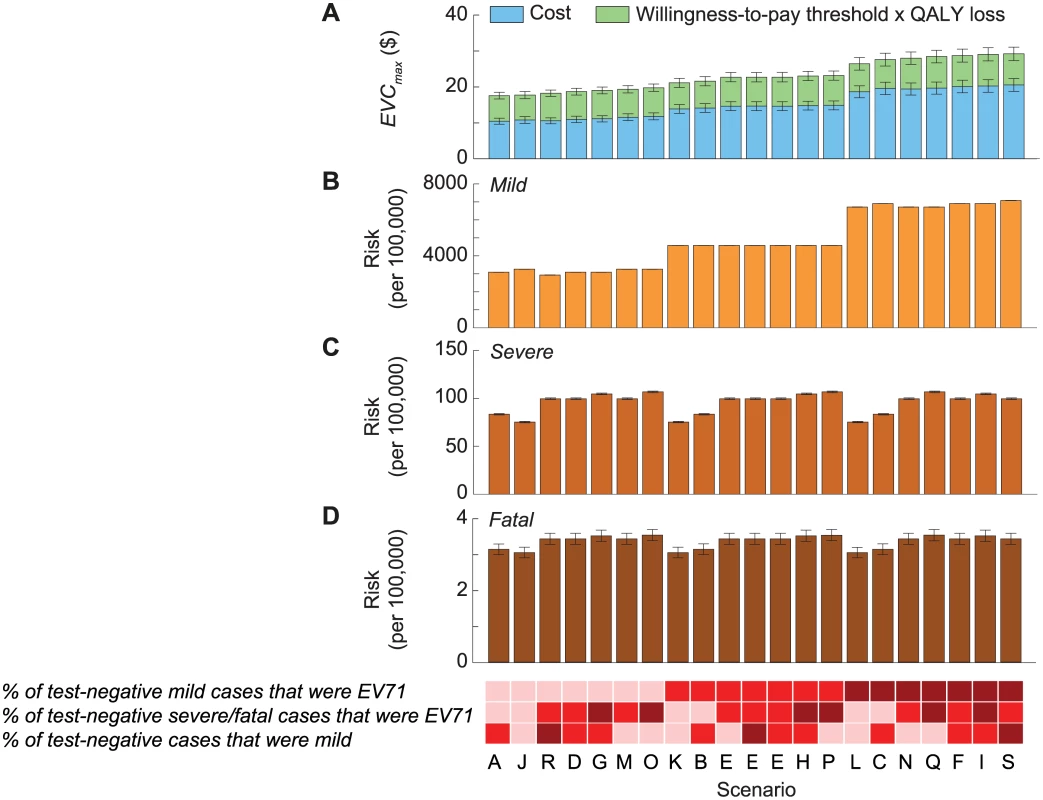
The 21 scenarios from Fig 2 are listed along the x-axis in ascending order of EVCmax. The base case was scenario A, which had the lowest EVCmax. The color shades at the bottom indicate the assumptions regarding the percentage of test-negative cases that were mild during 2010–2012 (bottom row), the percentage of test-negative severe/fatal cases that were EV71-HFMD (middle row), and the percentage of test-negative mild cases that were EV71-HFMD (top row) in each scenario, where darker shades correspond to higher percentages. As described in Fig 2B, three combinations of these percentages resulted in the same scenario, namely scenario E. The size of the 95% prediction intervals was driven by the parametric uncertainty associated with our estimates of the risk of EV71-HFMD and the mean cost and QALY loss attributed to EV71-HFMD in our probability sensitivity analysis (as in Fig 2B). (A) Pediatric EV71 vaccination would be cost-saving and cost-effective if EVC was below the cost of EV71-HFMD (blue bars) and EVCmax (blue plus green bars), respectively. (B–D) The risk of mild, severe, and fatal EV71-HFMD in each scenario. The error bars show the 95% CIs, but in some cases they are not apparent for the risk of mild and severe EV71-HFMD. Tab. 2. Cost-effectiveness ceiling on effective vaccine cost if all EV71-HFMD cases were registered by the national HFMD surveillance. 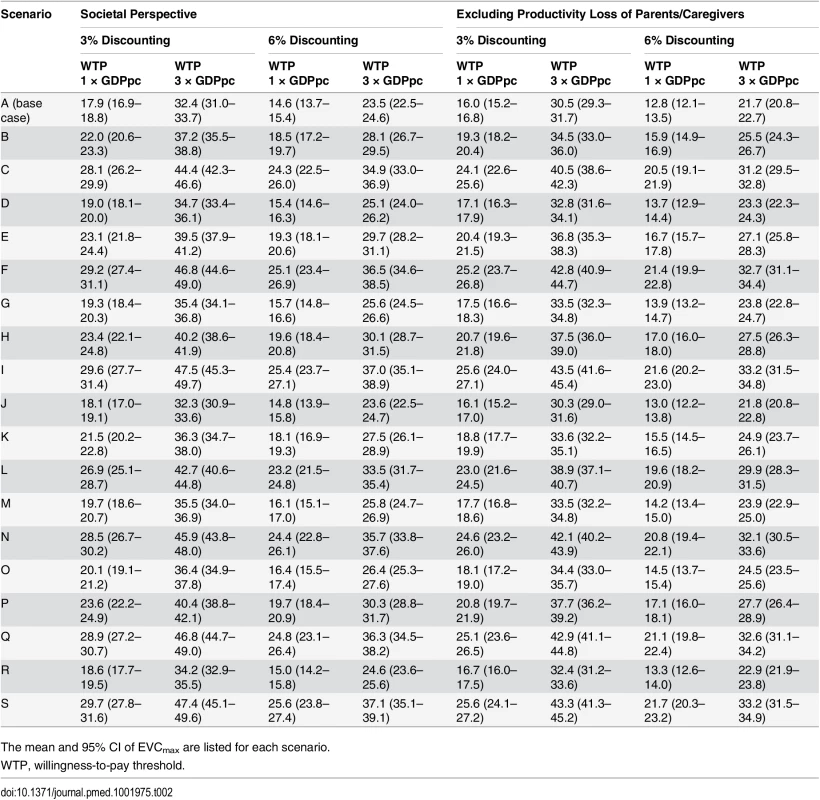
The mean and 95% CI of EVCmax are listed for each scenario. When productivity loss of parents/caregivers was excluded, EVCmax was 10%–14% lower (Tables 2 and S5; S2 Fig). EVCmax was very sensitive to the willingness-to-pay threshold. Increasing the threshold from one to three times GDPpc increased EVCmax by 58%–84% (Tables 2 and S5; S2 Fig).
EVCmax was sensitive to discount rate because QALY loss due to pediatric premature death—which was the major driver for health utility loss and a major constituent of EVCmax (Fig 3B and 3C)—was very sensitive to the discount rate. Increasing the discount rate for cost and health utility from 3% to 6% reduced EVCmax by around 14%–19% (Tables 2 and S5; S2 Fig).
We estimated that the aggregated incidence rate of EV71-HFMD in the EV71 vaccine phase III trials and the corresponding rate in national surveillance were 18.3 (95% CI 16.3–20.6) and 13.4 (95% CI 12.4–14.5) per 1,000 person-years, respectively. These estimates suggest that only 74% (95% CI 64%–84%) of EV71-HFMD cases in these areas were registered by national surveillance during the trial periods. If this proportion is generalizable to the whole country and the expected cost and QALY loss of registered and unregistered cases are similar, then EVCmax is increased by 36% (95% CI 23%–50%) (Tables 2 and 3; S3 Fig).
Tab. 3. Cost-effectiveness ceiling on effective vaccine cost if the proportion of EV71-HFMD cases registered by national surveillance was the same as that observed in the three EV71 vaccine trials. 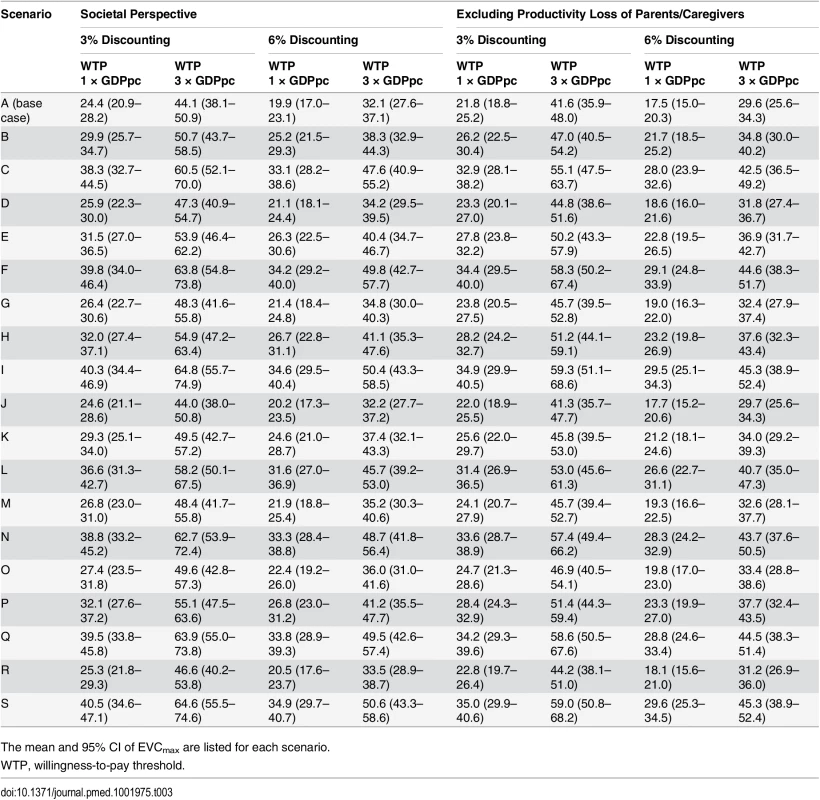
The mean and 95% CI of EVCmax are listed for each scenario. The efficacy estimates for the vaccines made by Beijing Vigoo Biological, Sinovac Biotech, and the Chinese Academy of Medical Sciences (CAMS) were 90% (95% CI 67.1%–96.9%), 94.8% (95% CI 87.2%–97.9%), and 97.4% (95% CI 92.9%–99%), respectively [12–14]. Using the base-case EVCmax, we estimated that EV71 vaccination would be cost-effective in China if the vaccine cost was below US$12.0–US$17.7, US$15.3–US$18.0, and US$16.2 – US$18.3 for the Beijing Vigoo Biological, Sinovac Biotech, and CAMS vaccines, respectively, when compared to no vaccination. Fig 5 indicates the optimal vaccine given any combination of the costs for the three vaccines within these ranges. For example, if all three vaccines were priced at the point estimate of EVCmax × vaccine efficacy (which corresponds to US$16.1, US$17.0, and US$17.4 for the Beijing Vigoo Biological, Sinovac Biotech, and CAMS vaccine, respectively), then the CAMS vaccine would be optimal.
Fig. 5. Comparative cost-effectiveness analysis of the three vaccine candidates based on the base-case EVCmax. 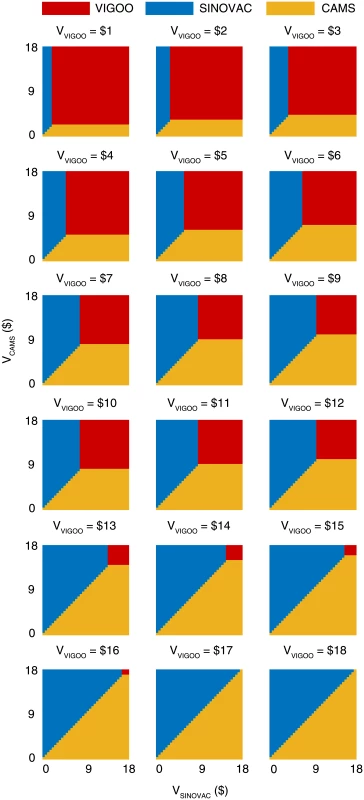
The color coding indicates the optimal vaccine given the costs of the three vaccines (VVigoo, VSinovac, VCAMS). Discussion
Our CEA suggests that routine pediatric EV71 vaccination in China would be very cost-effective if the all-inclusive EVC (including all logistical, procurement, and administration costs needed to confer 5 y of vaccine protection) is below US$17.9 (95% CI US$16.9–US$18.8). Compared to no vaccination, routine pediatric EV71 vaccination would be cost-effective in China if the vaccine cost is below US$12.0–US$17.7, US$15.3–US$18.0, and US$16.2–US$18.3 for the Beijing Vigoo Biological, Sinovac Biotech, and CAMS vaccines, respectively. Given any combination of the costs for the three vaccines within these ranges, policymakers can use Fig 5 to determine the optimal vaccine.
Our estimates of cost-effective vaccine cost ceiling are very conservative for the following reasons. First, we have likely underestimated the true disease burden of EV71, and hence EVCmax, because (i) EV71 can cause diseases other than HFMD [9] and (ii) underreporting and under-ascertainment of EV71-HFMD, as we have demonstrated, might be substantial (Tables 2 and 3; S2 and S3 Figs).
Second, we assumed that EV71 vaccination had no effect in reducing the severity of EV71-HFMD because there were too few severe cases in the phase III trials to provide robust evidence for such efficacy [12–14]. If EV71 vaccines, like many other vaccines such as rotavirus vaccines [23], can reduce disease severity, then we have underestimated EVCmax.
Third, we used one times GDPpc as the base-case willingness-to-pay threshold, in keeping with the WHO-CHOICE criterion for very cost-effective interventions [20]. The commonly used thresholds in high-income countries, such as the UK (£20,000–£30,000) [24], are close to one times GDPpc. WHO-CHOICE classifies interventions as cost-effective if the ICER is between one and three times GDPpc. This range of willingness-to-pay threshold might also be suitable for China, which is a middle-income country with a rapidly increasing GDPpc. As shown, EVCmax would increase by 60%–80% if the threshold was three times GDPpc (S2 Fig). On the other hand, there have been ongoing discussions about the appropriateness of the WHO-CHOICE thresholds, and some preliminary work suggests a real-world threshold of around 0.18–0.71 times GDPpc in middle - and high-income countries [25]. As an illustration, adopting a threshold of 0.5 times GDPpc would reduce EVCmax by around 25%.
Finally, we did not account for productivity loss due to pediatric premature death because of unresolved debate about the way such losses should be costed [18]. Including this productivity loss would increase EVCmax.
Our study has several limitations. First, we have presented our CEA results using EVC, an outcome with which readers may be less familiar compared to other outcomes commonly used in health economic evaluation, such as ICERs. The calculation underlying EVC, however, is the same as that for evaluating the incremental net monetary benefits of vaccination. We used EVC as the outcome because (i) health policymakers in China are currently most interested in the vaccine cost ceiling that would render routine pediatric EV71 vaccination cost-effective in order to prepare for a potential future addition to the Expanded Programme on Immunization and price negotiation with the vaccine manufacturers, and (ii) the reported vaccine efficacy and associated uncertainty for the three EV71 vaccine candidates are significantly different. As such, EVC, which is simply the vaccine cost adjusted for the corresponding vaccine efficacy, provides a succinct way of defining the vaccine cost ceiling without the need to reference the efficacy of any particular vaccine. As we have shown, EVC can be easily used to compare the cost-effectiveness of the three vaccine candidates, which have significantly different vaccine efficacy and hence vaccine cost ceilings.
Second, we have not accounted for the potential impact of an increase in the age of infection caused by herd immunity or limited duration of vaccine protection. Our previous study showed that the severity of EV71-HFMD decreases substantially with age [6]. Therefore, the cost-effectiveness of pediatric vaccination would be only slightly reduced if some of the burden averted by vaccination among children under 5 y is offset by increased infection in older age groups.
Third, we used self-reported hospital charges as a proxy for the opportunity cost of care, because of the difficulty of accessing hospital records across the entire country. Several studies have estimated the economic cost of HFMD treatment in single cities or counties in China using hospital records [26–34]. However, only two of these, one set in Ningbo in Zhejiang and the other in seven cities in Shandong, reported mean costs using hospital records and the same severity stratification as the national surveillance database [29,34]. The Ningbo study reported mean costs of US$161, US$530, and US$1,262, respectively, for mild outpatient, mild inpatient, and severe cases, whereas the estimates in the Shandong study were US$124, US$699, and US$2,076, respectively. In comparison, the corresponding costs in our study for the east region of China (where Ningbo and Shandong are located) were US$239, US$1,011, and US$3,592. The costs for mild cases in these two studies and ours differ by less than 48%, which suggests that our estimate of EVCmax should be reasonably robust because mild cases are the main cost driver (Fig 3C) and our CEA assumptions are very conservative.
Fourth, we modified the EuroQol EQ-5D-3L instument to elicit quality of life of children younger than 7 y, as has been done previously in similar studies [35]. Although such modifications have not been validated, the associated uncertainty had little effect on our results because 89% of QALY loss due to EV71-HFMD came from premature death (Fig 3C).
Fifth, we assumed that the cost and health utility loss estimates based on laboratory-confirmed EV71-HFMD cases were representative of all EV71-HFMD cases.
Sixth, we assumed that the long-term average risk of EV71-HFMD in the future would be similar to that registered by national surveillance during 2010–2013. Year-to-year variations in EV71-HFMD burden can be significant, and future EV71-HFMD epidemiology may not be congruent with this assumption. The confidence intervals associated with our estimates of EVCmax do not reflect possible deviations of future EV71-HFMD burden from the long-term average that we have assumed. For example, if future long-term EV71-HFMD were more similar to that in 2012 or 2013, which were the years with the highest and lowest EV71-HFMD burden, then EVCmax would be increased by 42% and decreased by 47%, respectively.
Finally, our analysis assumed that no alternative preventive interventions are available to be used that could also reduce the burden of EV71-HFMD. If such interventions exist, this may affect the cost-effectiveness of vaccination. We conducted a literature review of the potential effectiveness of alternative interventions to prevent the spread of EV71 (see “Under What Conditions Would We Not Vaccinate Because a More Cost-Effective Alternative Intervention Exists?” in S1 Text for details). The review found only one relevant article, which suggested that the extreme infection control measures imposed during the 2003 SARS outbreak in Hong Kong reduced the incidence of HFMD by 57.2%. However, these reactive control measures, which involved territory-wide school closure, hand and respiratory hygiene awareness campaigns, and disinfection, are unlikely to be reproducible for prolonged periods outside a pandemic or highly urbanized setting. Nevertheless, if such a level of reduction in HFMD incidence is achievable without using vaccination, then EVCmax would be reduced by 57.2% to US$7.3.
To our knowledge, this is the first economic evaluation of EV71 vaccination since the EV71 vaccine phase III trial results were published in 2013–2014. In 2010, Lee et al. [36] used the national surveillance data from 2007–2009 and extrapolated US health care cost figures to China to forecast that with a willingness-to-pay to avert a disability-adjusted life year of three times GDPpc, pediatric EV71 vaccination would be cost-effective from a third-party payer perspective if vaccine cost per individual was US$25 and vaccine efficacy was ≥70% [36]. The assumed risk of EV71-HFMD in their study (0.04%–1%) was much lower than in ours (3%–8%; Fig 4B) because the disease burden registered by national surveillance has increased substantially since 2009. In their sensitivity analysis, Lee et al. found that when the risk of EV71-associated diseases was 5% (i.e., similar to that in the intermediate scenarios in Fig 4B), pediatric EV71 vaccination would be cost-saving if vaccine cost was US$50 and vaccine efficacy was 80% (i.e., an EVC of US$63). In contrast, pediatric EV71 vaccination would be cost-saving in the corresponding scenarios of our study only if EVC < US$12.2 (S2 Fig). Therefore, the discrepancies between the two studies are also due to differences in cost estimates.
Our study is also the first to our knowledge to highlight that the current practice of omitting test-negative results generates substantial uncertainty regarding the burden of EV71-HFMD (Fig 4B). Laboratory surveillance is the cornerstone for monitoring diseases caused by multiple strains of pathogens, such as HFMD, pneumococcal diseases, etc. In our base case (in which we assumed that none of the test-negative cases were EV71-HFMD), EV71 accounted for 26.0% and 64.3% of mild and severe/fatal HFMD cases, respectively, in China during 2010–2013. Policymakers can regard this as the maximal proportion of HFMD burden that is preventable by routine pediatric EV71 vaccination. If routine pediatric EV71 vaccination is implemented and vaccine coverage is high, robust laboratory surveillance of HFMD-causing enteroviruses will be critical for monitoring vaccine impact as well as investigating herd immunity, cross-protection, and serotype replacement (e.g., as demonstrated for pneumococcal conjugate vaccination [16,37,38]).
Our current and previous study [6] suggest that there are large geographical variations in the risk of EV71-HFMD (S4 Fig). Such variations are likely due to geographical differences in underlying epidemiologic factors, underreporting, and health care quality. If national, population-wide rollout is infeasible in the initial stages of routine pediatric EV71 vaccination, policymakers could consider prioritizing areas in which EV71 vaccination is most cost-effective.
Supporting Information
Zdroje
1. Chua KB, Kasri AR. Hand foot and mouth disease due to enterovirus 71 in Malaysia. Virol Sin. 2011;26 : 221–8. doi: 10.1007/s12250-011-3195-8 21847753
2. Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT, Tsai SF, et al. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med. 1999;341 : 929–935. doi: 10.1056/NEJM199909233411301 10498487
3. Hosoya M, Kawasaki Y, Sato M, Honzumi K, Kato A, Hiroshima T, et al. Genetic diversity of enterovirus 71 associated with hand, foot and mouth disease epidemics in Japan from 1983 to 2003. Pediatr Infect Dis J. 2006;25 : 691–694. doi: 10.1097/01.inf.0000227959.89339.c3 16874167
4. Nguyen NT, Pham HV, Hoang CQ, Nguyen TM, Nguyen LT, Phan HC, et al. Epidemiological and clinical characteristics of children who died from hand, foot and mouth disease in Vietnam, 2011. BMC Infect Dis. 2014;14 : 341. doi: 10.1186/1471-2334-14-341 24942066
5. Wu Y, Yeo A, Phoon MC, Tan EL, Poh CL, Quak SH, et al. The largest outbreak of hand; foot and mouth disease in Singapore in 2008: the role of enterovirus 71 and coxsackievirus A strains. Int J Infect Dis. 2010;14:e1076–e1081. doi: 10.1016/j.ijid.2010.07.006 20952237
6. Xing W, Liao Q, Viboud C, Zhang J, Sun J, Wu JT, et al. Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. Lancet Infect Dis. 2014;14 : 308–318. doi: 10.1016/S1473-3099(13)70342-6 24485991
7. World Health Organization Western Pacific Region. Emerging disease surveillance and response—hand, foot and mouth disease (HFMD). [cited 29 Jan 2015]. http://www.wpro.who.int/emerging_diseases/HFMD/en/.
8. Chinese Center for Disease Control and Prevention. National Population and Health Science Data Sharing Platform. [cited 29 Jan 2015]. http://www.phsciencedata.cn/Share/en/index.jsp.
9. Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010;9 : 1097–1105. doi: 10.1016/S1474-4422(10)70209-X 20965438
10. Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis. 2010;10 : 778–790. doi: 10.1016/S1473-3099(10)70194-8 20961813
11. World Health Organization Western Pacific Region. A guide to clinical management and public health response for hand, foot and mouth disease (HFMD). Geneva: World Health Organization; 2011.
12. Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, et al. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med. 2014;370 : 829–837. doi: 10.1056/NEJMoa1303224 24571755
13. Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med. 2014;370 : 818–828. doi: 10.1056/NEJMoa1304923 24571754
14. Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2013;381 : 2024–2032. doi: 10.1016/S0140-6736(13)61049-1 23726161
15. Yu H, Yang W, Varma JK. To save children’s lives, China should adopt an initiative to speed introduction of pneumonia vaccines. Health Aff (Millwood). 2012;31 : 2545–2553. doi: 10.1377/hlthaff.2011.1272
16. Takahashi S, Liao Q, Van Boeckel TP, Xing W, Sun J, Hsiao VY, et al. Hand, foot, and mouth disease in China: modelling epidemic dynamics of enterovirus serotypes and implications for vaccination. PLoS Med. 2016. doi: 10.1371/journal.pmed.1001958
17. The World Bank. Life expectancy at birth. [cited 29 Jan 2015]. http://data.worldbank.org/indicator/SP.DYN.LE00.IN.
18. Koopmanschap MA, Rutten FF, van Ineveld BM, van Roijen L. The friction cost method for measuring indirect costs of disease. J Health Econ. 1995;14 : 171–189. 10154656
19. Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276 : 1253–1258. 8849754
20. Tan-Torres Edejer T, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, et al. Making choices in health: WHO guide to cost-effectiveness analysis. Geneva: World Health Organization; 2003.
21. National Bureau of Statistics of China. National data: national accounts. [cited 29 Jan 2015]. http://data.stats.gov.cn/english/easyquery.htm?cn=C01.
22. Zhu FC, Liang ZL, Meng FY, Zeng Y, Mao QY, Chu K, et al. Retrospective study of the incidence of HFMD and seroepidemiology of antibodies against EV71 and CoxA16 in prenatal women and their infants. PLoS ONE. 2012;7:e37206. doi: 10.1371/journal.pone.0037206 22662137
23. Panozzo CA, Becker-Dreps S, Pate V, Weber DJ, Jonsson Funk M, Sturmer T, et al. Direct, indirect, total, and overall effectiveness of the rotavirus vaccines for the prevention of gastroenteritis hospitalizations in privately insured US children, 2007–2010. Am J Epidemiol. 2014;179 : 895–909. doi: 10.1093/aje/kwu001 24578359
24. National Institute for Health and Care Excellence. The guidelines manual: assessing cost effectiveness. 2012 Nov [cited 23 Mar 2015]. https://www.nice.org.uk/article/pmg6/chapter/7-assessing-cost-effectiveness.
25. Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. York: University of York Center for Health Economics; 2015.
26. Lu Y, Dong Y, Xu Y. Cost-effectiveness analysis of 4 therapeutic schemes for children herpangina. China Pharmaceuticals. 2011;20 : 48–50.
27. Tang X, Li W, Wang W. Cost-minimization analysis of three therapeutic schemes for hand-foot-mouth disease. China Mod Med. 2010;33 : 095.
28. Yang T. Cost-effectiveness analysis of medication treatment for HFMD in children. Chin J Mod Drug Appl. 2012;6 : 79–80.
29. He T-F, Yang T-C, Yi B, Xu G-Z. Economic burden estimation for pandemic hand foot and mouth disease in Ningbo City, 2011. Shanghai J Prev Med. 2012;9 : 001.
30. Yang T-C, Yi B, He T-F, Wu Y, Ma Y, Xu G. Economic burden of hand, foot and mouth disease in Ningbo, Zhejiang. Dis Surveill. 2012;27 : 520–523.
31. Qin Y, Zhang J, Zhang J, Mou G, Xing Y, Liu J. Investigation on economical burden and status of health care seeking among 1398 hand-foot-mouth disease cases in Yantai. Chin J Epidemiol. 2009;30 : 1319–1320.
32. Gan Z-K, Jin H, Li J-X, Yao X-J, Zhou Y, Zhang X-F, et al. Disease burden of enterovirus 71 in rural central China: a community-based survey. Hum Vaccin Immunother. 2015;11 : 2400–2405. doi: 10.1080/21645515.2015.1059980 26158689
33. Li W, Li H, Li G-F, Hu Y-H. Survey on economic burden of hand, foot and mouth disease in Huaiyin District of Jinan City 2012. Prev Med Trib. 2014;7 : 484–487.
34. Liu T. Research on economic burden of disease and its determinants of hand, foot and mouth disease patients in Shandong Province. Jinan: Shandong University; 2013.
35. Martin A, Cottrell S, Standaert B. Estimating utility scores in young children with acute rotavirus gastroenteritis in the UK. J Med Econ. 2008;11 : 471–484. doi: 10.3111/13696990802321047 19450099
36. Lee BY, Wateska AR, Bailey RR, Tai JH, Bacon KM, Smith KJ. Forecasting the economic value of an enterovirus 71 (EV71) vaccine. Vaccine. 2010;28 : 7731–7736. doi: 10.1016/j.vaccine.2010.09.065 20923711
37. Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11 : 760–768. doi: 10.1016/S1473-3099(11)70090-1 21621466
38. Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Liu B, Bateson D, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. Lancet Infect Dis. 2014;14 : 958–966. doi: 10.1016/S1473-3099(14)70841-2 25107680
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2016 Číslo 3- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Trans-Pacific Partnership Provisions in Intellectual Property, Transparency, and Investment Chapters Threaten Access to Medicines in the US and Elsewhere
- An Uninformative Truth: The Logic of Amarin’s Off-Label Promotion
- Performance of the GeneXpert Ebola Assay for Diagnosis of Ebola Virus Disease in Sierra Leone: A Field Evaluation Study
- Pragmatic Trials for Noncommunicable Diseases: Relieving Constraints
- Antibiotic Resistance in India: Drivers and Opportunities for Action
- Global Role and Burden of Influenza in Pediatric Respiratory Hospitalizations, 1982–2012: A Systematic Analysis
- Routine Pediatric Enterovirus 71 Vaccination in China: a Cost-Effectiveness Analysis
- Increased Duration of Paid Maternity Leave Lowers Infant Mortality in Low- and Middle-Income Countries: A Quasi-Experimental Study
- Planned Repeat Cesarean Section at Term and Adverse Childhood Health Outcomes: A Record-Linkage Study
- Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea
- A Novel Brief Therapy for Patients Who Attempt Suicide: A 24-months Follow-Up Randomized Controlled Study of the Attempted Suicide Short Intervention Program (ASSIP)
- Comparison of the Schwartz and CKD-EPI Equations for Estimating Glomerular Filtration Rate in Children, Adolescents, and Adults: A Retrospective Cross-Sectional Study
- Cardiovascular and Renal Outcomes of Renin–Angiotensin System Blockade in Adult Patients with Diabetes Mellitus: A Systematic Review with Network Meta-Analyses
- Length of Stay After Childbirth in 92 Countries and Associated Factors in 30 Low- and Middle-Income Countries: Compilation of Reported Data and a Cross-sectional Analysis from Nationally Representative Surveys
- Transformative Innovations in Reproductive, Maternal, Newborn, and Child Health over the Next 20 Years
- Compassionate and Proactive Interventions by Health Workers in the United Kingdom: A Better Approach to Prevent and Respond to Female Genital Mutilation?
- Translational Research for Tuberculosis Elimination: Priorities, Challenges, and Actions
- The Community As the Patient in Malaria-Endemic Areas: Preempting Drug Resistance with Multiple First-Line Therapies
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea
- Comparison of the Schwartz and CKD-EPI Equations for Estimating Glomerular Filtration Rate in Children, Adolescents, and Adults: A Retrospective Cross-Sectional Study
- Performance of the GeneXpert Ebola Assay for Diagnosis of Ebola Virus Disease in Sierra Leone: A Field Evaluation Study
- A Novel Brief Therapy for Patients Who Attempt Suicide: A 24-months Follow-Up Randomized Controlled Study of the Attempted Suicide Short Intervention Program (ASSIP)
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy





