-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
International Funding for Malaria Control in Relation to Populations at Risk of Stable Transmission
Background:
The international financing of malaria control has increased significantly in the last ten years in parallel with calls to halve the malaria burden by the year 2015. The allocation of funds to countries should reflect the size of the populations at risk of infection, disease, and death. To examine this relationship, we compare an audit of international commitments with an objective assessment of national need: the population at risk of stable Plasmodium falciparum malaria transmission in 2007.Methods and Findings:
The national distributions of populations at risk of stable P. falciparum transmission were projected to the year 2007 for each of 87 P. falciparum–endemic countries. Systematic online - and literature-based searches were conducted to audit the international funding commitments made for malaria control by major donors between 2002 and 2007. These figures were used to generate annual malaria funding allocation (in US dollars) per capita population at risk of stable P. falciparum in 2007. Almost US$1 billion are distributed each year to the 1.4 billion people exposed to stable P. falciparum malaria risk. This is less than US$1 per person at risk per year. Forty percent of this total comes from the Global Fund to Fight AIDS, Tuberculosis and Malaria. Substantial regional and national variations in disbursements exist. While the distribution of funds is found to be broadly appropriate, specific high population density countries receive disproportionately less support to scale up malaria control. Additionally, an inadequacy of current financial commitments by the international community was found: under-funding could be from 50% to 450%, depending on which global assessment of the cost required to scale up malaria control is adopted.Conclusions:
Without further increases in funding and appropriate targeting of global malaria control investment it is unlikely that international goals to halve disease burdens by 2015 will be achieved. Moreover, the additional financing requirements to move from malaria control to malaria elimination have not yet been considered by the scientific or international community.
Published in the journal: International Funding for Malaria Control in Relation to Populations at Risk of Stable Transmission. PLoS Med 5(7): e142. doi:10.1371/journal.pmed.0050142
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.0050142Summary
Background:
The international financing of malaria control has increased significantly in the last ten years in parallel with calls to halve the malaria burden by the year 2015. The allocation of funds to countries should reflect the size of the populations at risk of infection, disease, and death. To examine this relationship, we compare an audit of international commitments with an objective assessment of national need: the population at risk of stable Plasmodium falciparum malaria transmission in 2007.Methods and Findings:
The national distributions of populations at risk of stable P. falciparum transmission were projected to the year 2007 for each of 87 P. falciparum–endemic countries. Systematic online - and literature-based searches were conducted to audit the international funding commitments made for malaria control by major donors between 2002 and 2007. These figures were used to generate annual malaria funding allocation (in US dollars) per capita population at risk of stable P. falciparum in 2007. Almost US$1 billion are distributed each year to the 1.4 billion people exposed to stable P. falciparum malaria risk. This is less than US$1 per person at risk per year. Forty percent of this total comes from the Global Fund to Fight AIDS, Tuberculosis and Malaria. Substantial regional and national variations in disbursements exist. While the distribution of funds is found to be broadly appropriate, specific high population density countries receive disproportionately less support to scale up malaria control. Additionally, an inadequacy of current financial commitments by the international community was found: under-funding could be from 50% to 450%, depending on which global assessment of the cost required to scale up malaria control is adopted.Conclusions:
Without further increases in funding and appropriate targeting of global malaria control investment it is unlikely that international goals to halve disease burdens by 2015 will be achieved. Moreover, the additional financing requirements to move from malaria control to malaria elimination have not yet been considered by the scientific or international community.Introduction
The Global Fund to Fight AIDS, Tuberculosis and Malaria (GFTAM) [1–3] was established in January 2002 as an independent financing body to attract, manage, and disburse funds to control these three major diseases of poverty. This innovative mechanism for results-based health care financing had by the end of 2007 committed US$10 billion to 136 countries [1,2]. The GFATM responds to nationally documented demand for antimalarial interventions and commodities. Countries are encouraged to submit proposals every year, which are reviewed independently by a technical review panel before the GFATM board makes a decision [4,5]. The GFATM state that their funding priorities are to countries/regions with the highest disease burdens and weakest financial capacity to support disease control [1,6]. By the end of 2006 GFATM support represented an estimated 64% of all international funding for malaria control worldwide [6,7]. Rather than displace international support for malaria, new funding initiatives have emerged in recent years parallel to the GFATM, notably the World Bank global strategy and booster program [8] and the US President's Malaria Initiative (PMI) [9]. Bilateral agencies also continue to expand their support to countries as part of combined efforts to meet the Millennium Development Goals (MDG) [10,11]. As the funding capacity for global malaria control increases, it is important to examine the allocation of financing in relation to the distribution of populations most at risk of the disease; that is, to measure the equity of disbursements.
A perennial problem facing needs-based allocation of resources for malaria is the quantification of requirements based upon reliable descriptions of national populations at risk of infection and disease burden [12]. We recently published an evidence-based global distribution map of malaria risk that shows the most precise and contemporary description of the spatial limits of stable and unstable P. falciparum risk [13]. In the present study we use descriptions of stable malaria risk to categorize the biological vulnerability of P. falciparum malaria–endemic countries (PfMECs) and examine the per capita financial contributions approved by the GFATM, domestic funding, and support from other donors.
Methods
Defining Populations at Risk of P. falciparum Malaria in 2007
The contemporary spatial distribution of P. falciparum malaria and populations at risk of P. falciparum malaria (PfPAR) are described in detail elsewhere [13]. In brief, data on national case reporting, national and international medical intelligence, climate, and aridity were used to iteratively, and conservatively, define the margins of stable and unstable P. falciparum transmission globally. A definition of stable malaria of a minimum average of one clinical case per 10,000 population per annum (p.a.) in a given administrative unit was used. The historical definitions of stable and unstable malaria are rarely measured, as they are entomologically based metrics. The revised stable–unstable classification of P. falciparum Annual Parasite Incidence was based on a review [14] of the statistical, logistical, programmatic, and pragmatic reasons underpinning the levels used to define action points during the Global Malaria Eradication Programme [14–18]. This definition allows the use of widely available surveillance data that provide a measurable guide to the frequency of malaria exposure at a global level [14]. No transmission was assumed where assembled intelligence stated no malaria risk, because (i) not a single P. falciparum clinical case had been reported to national reporting systems over several years, or (ii) where temperature was too low for sporogony to complete within the average life span of the local dominant vector species, or (iii) conditions were too arid for anopheline mosquito survival. Unstable malaria was used to define areas where transmission was biologically plausible and/or had been documented but where incidence was likely to be less than one case per 10,000 population p.a. [14–18]. We estimated that the global population at any risk of P. falciparum transmission in 2007 was 2.37 billion across 87 countries and included 0.98 billion people living in areas defined as low, unstable transmission risk (see Figure 1).
Fig. 1. P. falciparum Malaria Risk Defined by Annual Parasite Incidence, Temperature, and Aridity 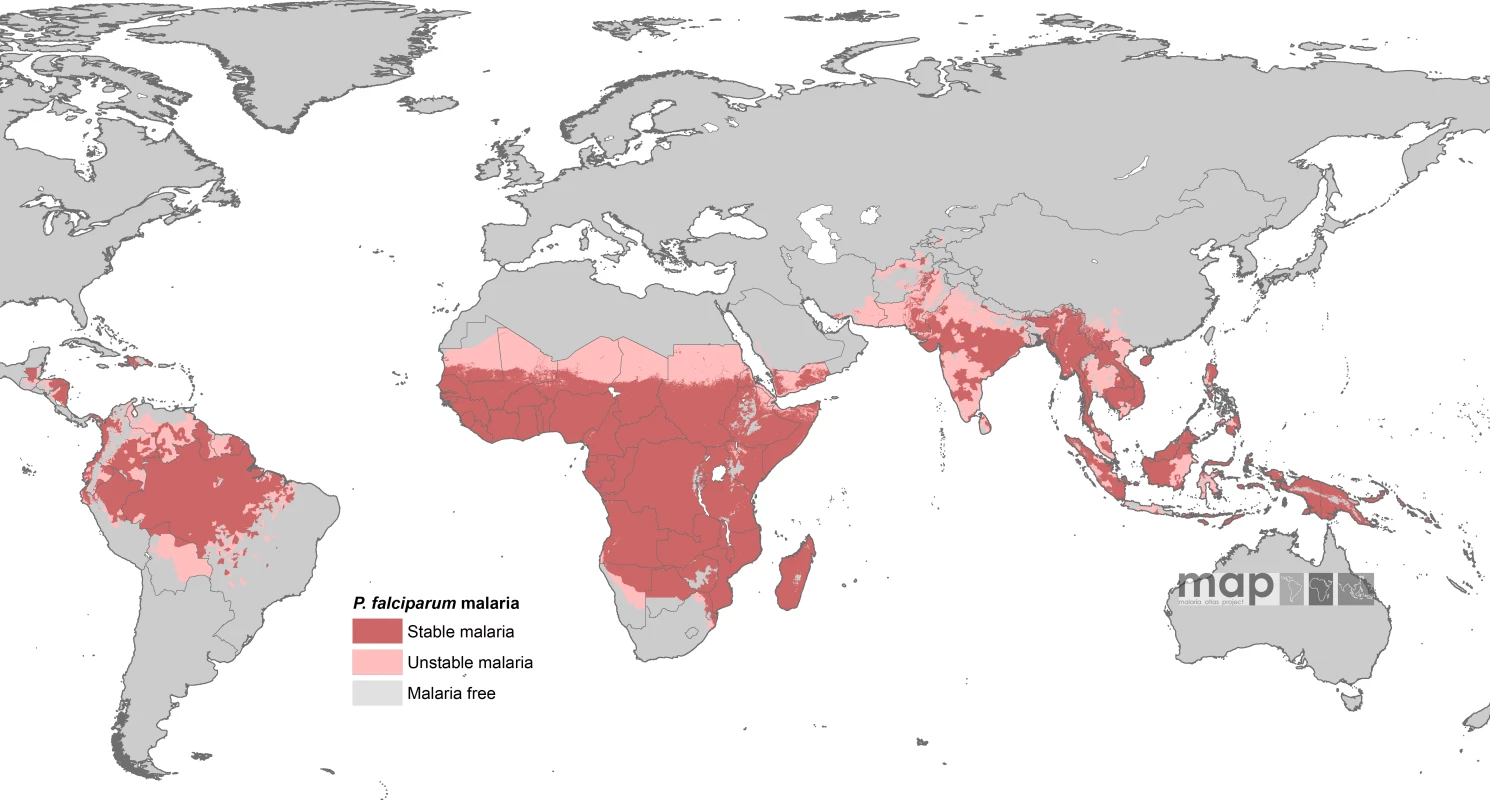
Populations at risk in areas defined as having stable (dark pink) and unstable transmission (light pink) were extracted for each of the 87 PfMECs. From [13]. Country-level extractions of the population densities residing in unstable and stable P. falciparum–endemic areas were undertaken using the Global Rural Urban Mapping Project (GRUMP) alpha version that provides gridded population counts and population density estimates for the years 1990, 1995, and 2000, both adjusted and unadjusted to the United Nations' national population estimates [19]. We used the adjusted population counts for the year 2000 and projected them to 2007 by applying national, medium variant, intercensal growth rates by country [20] using methods previously described [21]. This resulted in a contemporary global population surface and a 1 × 1 km spatial resolution, describing populations living in unstable and stable P. falciparum–endemic areas in each of the 87 PfMECs. The populations at risk were then calculated by overlaying the malaria risk map on the population surface in a geographic information system (ArcView GIS 3.2, ESRI, 1999).
We used populations at any risk of P. falciparum malaria infection and those living in areas of stable transmission as two strata of malaria risk in each country. Stable risk represents a more realistic estimate of populations at risk of significant disease burdens but does not distinguish between those populations exposed to infrequent malaria infection risks and those subject to repeated high infection and thus high disease burden risks [14,22]. In the present analyses, emphasis is given to populations at risk of stable P. falciparum malaria (PfPARstable), as they have the greatest public health needs that can be addressed using combinations of currently proven interventions and are the focus of the Roll Back Malaria (RBM) initiative since 1998 [23,24]. The GFATM uses eight regional groupings in its presentation of country applications; we have adapted these categories by collapsing the regions into four groupings: Africa, South East Asia/Western Pacific, Middle East/Eastern Europe, and the Americas/Caribbean (the countries in each region are defined in Table S1 and the footnote to Table 1).
Tab. 1. 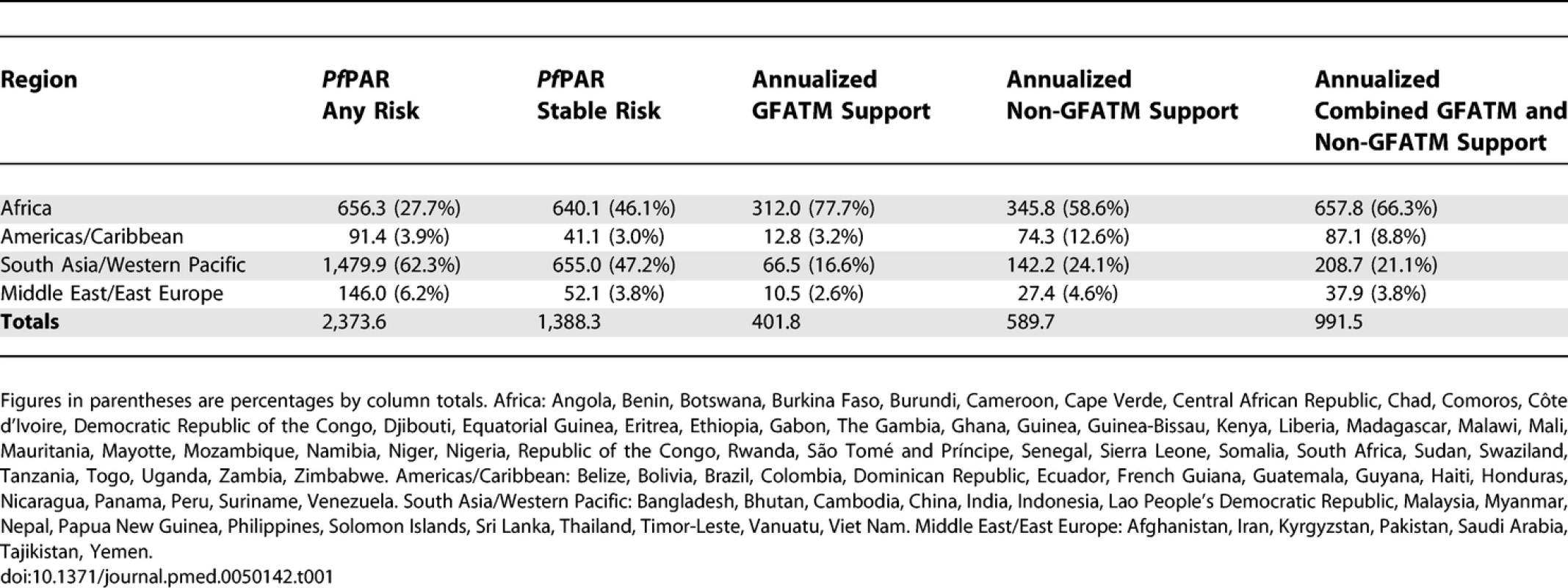
Regional Populations (Millions) at Any and Stable P. falciparum Risk in 2007, Annualized GFATM Approved Funding (Millions, US Dollars) and Annualized Non-GFATM Malaria Funding (Millions, US Dollars) We have not developed a similar risk map for the spatial extents of P. vivax. There are important differences in the biology of P. vivax transmission [25,26], the skill with which the parasite can be diagnosed clinically [27], and a less well defined relationship between transmission intensity and disease outcome. These factors all make an informed cartography and modelling of P. vivax distribution considerably more complex than for P. falciparum. We do not underestimate the likely disease burden of P. vivax malaria [28,29] and recognize that the global extent and public health consequences of this parasite remain inadequately defined. It is with this caveat that we have used the risks of the most clinically important and best-mapped human malaria parasite, P. falciparum, to articulate biological needs for malaria control investment. In addition, since P. falciparum is easier to control [14–18], dealing with this parasite is more often regarded as a national and international priority.
GFATM-Approved Funding for Malaria Control 2002–2007
We have focused on approved funding between round one (2002) and round seven (2007), rather than on signed contracts or disbursed funds. Approved funding is the expressed needs of country or region in applications that have been reviewed for technical content and approved by the GFATM. Not all funding has been disbursed and not all contracts would have been signed by the end of 2007; these are difficult to reconcile between rounds of GFATM funding and between countries. Therefore our focus was on the funds approved by the GFATM under the assumption that these approved funds reflect need and do not capture the idiosyncrasies of delayed funding disbursement or cessation of funding due to poor performance or governance [30]. This therefore represents the ability of the GFATM to respond to need, rather than the ability of a government to deliver.
All approved proposals that included a malaria component were assembled from the GFATM Web site [3]. It was difficult to attribute funding to individual countries uniquely where support was awarded for regional initiatives. Such multi-country proposals included those in southern Africa (South Africa, Mozambique, and Swaziland), the Andean region in the Americas (Colombia, Ecuador, Peru, and Venezuela), and the Western Pacific region (Solomon Islands and Vanuatu). In these cases we assumed a proportional allocation by country PfPAR relative to the total PfPAR for the region (e.g., PfPAR estimated for Ecuador is 18.8% of the total PfPAR for all four Andean countries combined [5.8 million/30.8 million people]; hence funds assumed for Ecuador were 18.8% of the total funds of the proposal).
Clearly, the approval of awards for malaria by the GFATM has been variable across the 5-y interval. During round one very few malaria awards were approved, representing only 14% of all funds approved in 2002 [31]. In contrast, during round four, a total of US$631 million was awarded to 22 countries. In addition, excluding regional submissions, 21 countries had only a single award approved between rounds one and seven, 35 countries were approved in at least two submissions, and ten countries were approved funding in three or more rounds. Without trying to weigh each timed award per country we have aggregated the combined awards across all approved rounds (one to seven) to represent a single GFATM commitment to each PfMEC and taken an annual average of this figure over the 6 y to reflect the averaged approved funding for malaria control per country p.a. This annual estimate has no temporal midpoint, as funds awarded in 2007 have yet to have signed contracts or be disbursed and include the 5 y post-2007, and are simply a p.a. commitment quantified over the number of years of existing funding rounds.
Defining Additional National and External Support for Malaria Control by Country
The GFATM seeks not to displace existing national-level funding for malaria control [1–3], rather to provide additional resources to meet internationally agreed targets. The World Malaria Report (WMR) produced by WHO and UNICEF [32] provides information on the amount of money spent by national governments on malaria control and prevention each year since 1998. These funds are domestic commitments or expenditure on malaria reported by governments to the WHO and converted to US dollars using the contemporary official exchange rate. These data have been used by others to estimate unmet financial needs for malaria control [33], and here we have assumed that the most recent domestic funding figure provided in the WMR is a reflection of (but not the actual) national financial commitment to malaria control. Data for 23 countries were not available in the WMR, and we assumed that these countries were unable or unwilling to provide these data. The wide variation in reported commitments per capita between countries precluded the use of a neighbouring country average. We chose to assume that countries not reporting a domestic funding commitment provided funds equivalent to the lowest rounded figures in their respective regions as follows: Africa US$30,000 p.a.; South East Asia/Western Pacific US$50,000 p.a.; Middle East/Eastern Europe US$100,000 p.a., and the Americas/Caribbean US$100,000 p.a. (Table S1).
Additional malaria-specific support is provided to countries as direct grants or, in the case of the World Bank, as very low interest long-term loans. These additional funding partners include bilateral and multilateral agencies: the UK's Department for International Development (DFID) supporting Kenya, Nigeria, and Mozambique [34]; the United States Agency for International Development (USAID), which provides recipient country data on its Web site [35]; and the PMI, launched by US President G. W. H. Bush in June 2005 with the aim of spending US$1.2 billion in 15 African countries over 5 y [9]. During the first phase of PMI activities in 2005, three countries were included in the programme (Angola, Tanzania, and Uganda); funding and activities began in an additional four countries in 2007 (Mozambique, Senegal, Malawi, and Rwanda), and although initial consultations have been completed in Benin, Ghana, Mali, Liberia, Ethiopia, Madagascar, Zambia, and Kenya, there are no details of proposed funding allocations for these countries. The World Bank global strategy and booster program has invested US$432 million in 15 countries since 2005, including two new projects approved in Mozambique and Kenya for 2007–2008 [8] and a multi-country, multi-sector programme covering the Senegal River Basin (Senegal, Mali, Mauritania, and Guinea) which was difficult to audit in terms of malaria-specific and country-specific allocations and was therefore excluded from the final analysis (US$42 million for the whole program). Additional World Bank support for periods of between 5 and 7 y from 2002 was assumed as described by Narasimhan and Attaran [36] for ten countries.
Many other important initiatives provide additional support through humanitarian sponsorship or tariffs for commodities [37–39], as part of technical assistance [40], humanitarian assistance [41], broader development assistance from regional banks [42–44], and nongovernment support by varied international and regional NGO partnerships in-country [45–47]. These alternative funding sources were explored through Web searches; however, none of these initiatives provided adequate detail on project–country-specific funding or commodity distribution to enable a comprehensive analysis of committed finances. There were two important exceptions: the Bill & Melinda Gates Foundation US$35 million donation to PATH to support comprehensive malaria control in Zambia between 2005 and 2014 [48] and the Arabian peninsula countries' 2007 announcement that they would provide US$17 million to Yemen over 5 y to implement its malaria elimination plans [49].
We used information from the World Bank, DFID, USAID, PMI, the domestic funding estimates in the WMR, and the Gates Foundation and Arabian donations to compute a non-GFATM estimate of financial support for malaria control. Data from bilateral, multilateral, or donation support were assembled over multi-year periods between 2004 and 2007 but aggregated to an average in US dollars over the reporting period per donor commitment p.a. per country.
Results
Regional P. falciparum Risks in Relation to Overall GFATM and Non-GFTAM Funding
The vast majority of people living at any risk of P. falciparum transmission worldwide are in South East Asia/Western Pacific (62.3%), followed by Africa (27.7%), the Middle East/East Europe (6.1%), and the Americas/Caribbean (3.8%; Table 1). The numbers, and relative proportion, of the global population at P. falciparum risk living in areas of stable endemic malaria are different (Table 1); values are almost equal in Africa (46.1%) and South East Asia/Western Pacific (47.2%). Total estimated PfPAR by country ranged from 58,568 people in Suriname to 950 million in India. PfPARstable ranged from approximately 8,000 (Suriname) and 19,000 (Djibouti) to between 130,000 (São Tomé and Príncipe) and 140,000 (French Guiana and Guyana) and to approximately 30–415 million people in eight countries (Pakistan, Tanzania, Myanmar, Ethiopia, the Democratic Republic of Congo, Indonesia, Nigeria, and India). Five countries had no populations living under conditions of stable transmission (Belize, Cape Verde, Mayotte, Tajikistan, and Kyrgyzstan; Table S1).
Seventy-seven countries had been approved for funding for malaria control by the end of 2007, including Georgia, where malaria transmission involves only P. vivax and is excluded from the analysis of funding presented here. Table S1 provides country-level estimates of the annual GFATM–approved funding per capita population living in areas of any or stable P. falciparum risk from 2002 to 2007. Seventy-six of the 87 PfMECs had been approved funding by round seven, either directly (n = 69) or through regional applications (n = 7), amounting to a total of US$2.41 billion through 133 separate grant applications since round one. Eleven PfMECs have never been awarded GFATM support for malaria (Belize, Botswana, Brazil, Cape Verde, the Democratic Republic of Congo, Dominican Republic, French Guiana, Mayotte, Malaysia, Panama, and Saudi Arabia). Three of these countries have zero PfPARstable (Belize, Cape Verde, and Mayotte). Approximately 26.5 million people are at risk of stable transmission in the remaining countries not receiving funding. Total funds approved to countries by the GFATM in a single round ranged between US$1.5 million and US$84.5 million (Table S1). On average, populations living in stable, endemic areas of the world have been approved funding by the GFATM at US$2.9 per capita-at-risk p.a. since 2002. African populations under stable transmission have been awarded US$1.5 annually per capita-at-risk, the Americas/Caribbean US$10.3 per capita-at-risk p.a., Middle East/East Europe US$1.1 per capita-at-risk p.a. and the lowest per capita commitment noted in South East Asia/West Pacific (US$0.7 per capita-at-risk p.a.).
Of the 74 countries with populations exposed to stable endemic transmission that have been allocated malaria funds by the GFATM, the average annual per capita at stable risk awarded amounts ranged from US$0.01 in Myanmar to US$147 in Suriname. Twenty-four countries had an average annual award of more than US$1 per capita at stable risk p.a., 12 were located in Africa and these were predominantly smaller countries (São Tomé and Príncipe, Burundi, Rwanda, Djibouti, The Gambia, Gabon, Equatorial Guinea, and Swaziland [Figure 2; Table S1]). Only seven countries had average annual awarded funding exceeding US$4 per capita at stable risk (São Tomé and Príncipe, Djibouti, Equatorial Guinea, Guyana, Venezuela, Iran, and Suriname [Figure 2; Table S1]).
Fig. 2. Mean Approved GFATM Funding for 87 PfMECs Expressed as US Dollars (USD) Per Capita at Stable P. falciparum Risk Per Annum 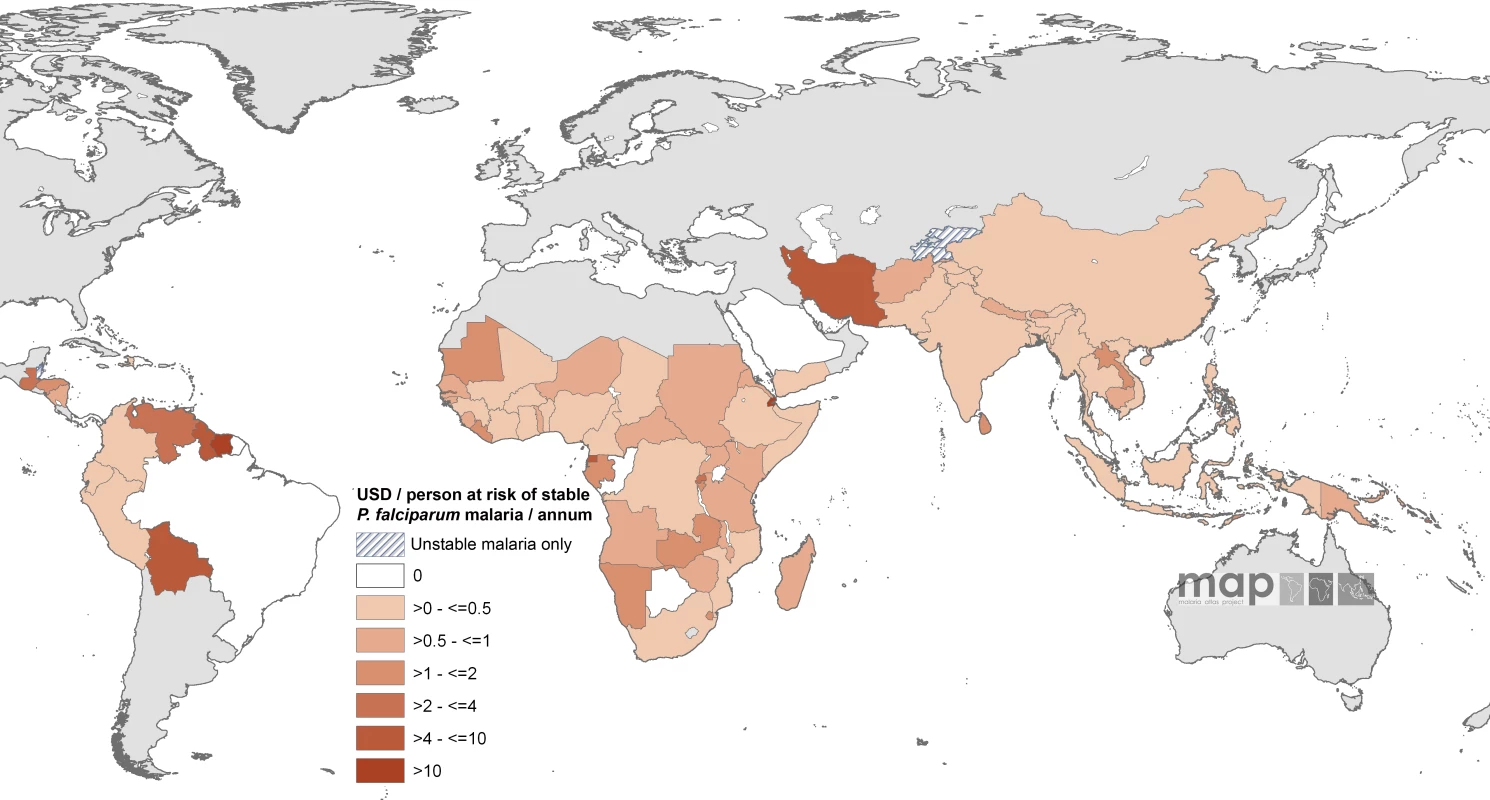
Countries indicated by hatching are those with no areas of stable risk. The enumerated non-GFATM malaria funding to each country amounted to US$589.8 million on an average year between 2002 and 2007, approximately 59% of the estimated combined GFATM and non-GFATM annualized commitments. The non-GFATM funds and their sources are shown by country in Table S1. On an average annual per capita at stable risk basis, the least non-GFATM support per person was documented in the Philippines (US$0.0023) and the highest amounts in Saudi Arabia (US$19.7), Suriname (US$20.3), and Iran (US$42.4). Twenty-one countries had an average of more than US$1 non-GFATM per capita support for malaria, seven countries had a non-GFATM annual per capita-at-stable risk commitment in excess of US$4; none were located in Africa (Bolivia, Guyana, Iran, Panama, Saudi Arabia, Suriname, and Venezuela; Figure 3; Table S1).
Fig. 3. Mean Approved Non-GFATM Funding for 87 PfMECs Expressed as US Dollars (USD) Per Capita at Stable P. falciparum Risk Per Annum 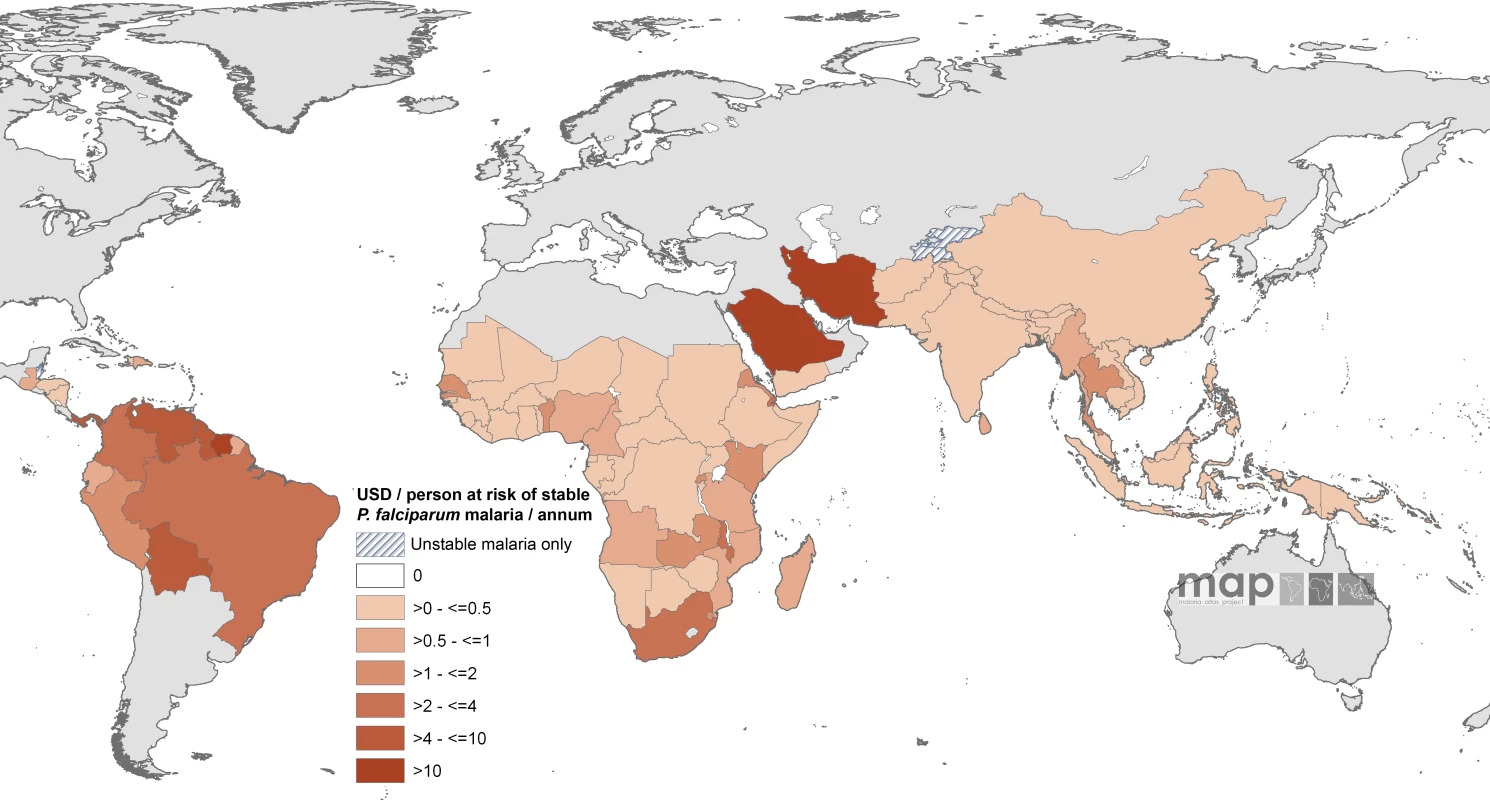
Countries indicated by hatching are those with no areas of stable risk. When combined with GFATM per capita support to populations in stable endemic areas, ten countries had an estimated combined per capita annual commitment of more than US$4 per person in an average year (Equatorial Guinea, Panama, São Tomé and Príncipe, Guyana, Bolivia, Venezuela, Saudi Arabia, Djibouti, Iran, and Suriname [Figure 4; Table S1]) compared to 34 countries where the combined annual commitment was less than US$1 per capita at stable risk, including 16 countries where annual malaria support was less than US$0.5 (Figure 4). These 16 countries encompass approximately 710 million people living under conditions of stable transmission, or 50% of the global population exposed to these risks of malaria transmission, and include seven of the poorest countries in Africa (Côte d'Ivoire, Republic of the Congo, Chad, Mali, Democratic Republic of the Congo, Somalia, and Guinea) and two of the most densely populated stable endemic countries in the world (Indonesia and India).
Fig. 4. Mean Approved Combined GFATM and Non-GFATM Funding for 87 PfMECs Expressed as US Dollars (USD) Per Capita at Stable P. falciparum Risk Per Annum 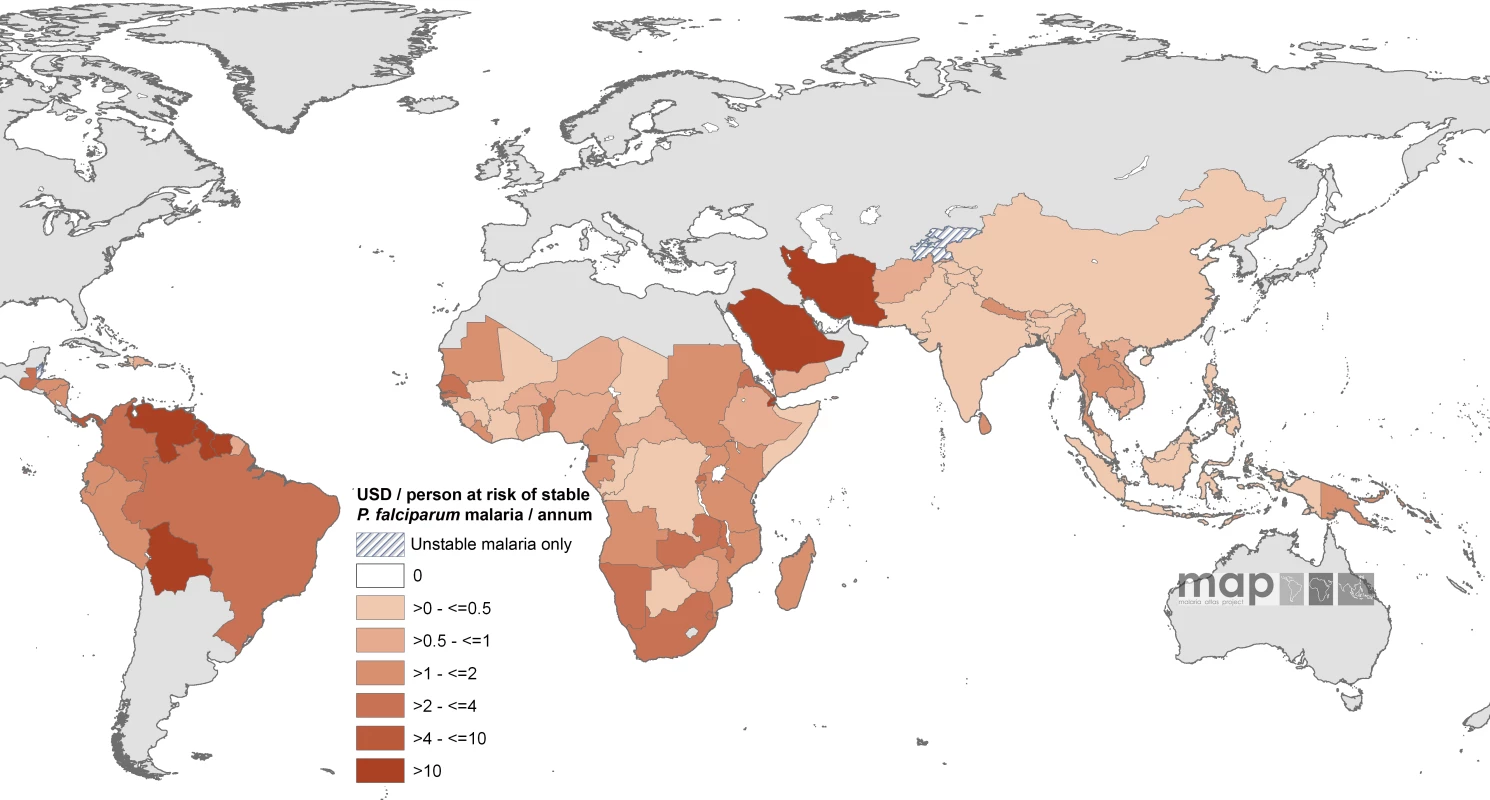
Countries indicated by hatching are those with no areas of stable risk. Discussion
Global and Regional Need for Malaria Control Versus Financial Disbursement
Plasmodium falciparum malaria continues to cause a huge global disease burden with an estimated 0.5 billion clinical episodes [22] and is the direct cause of over a million deaths in Africa each year [50,51]. The international community has renewed its commitment to its control as a specific component of the MDGs [10,11] and are reconsidering prospects for its elimination in areas where feasible [52,53]. The ten years since the launch of the RBM initiative [24,54] have witnessed a substantial increase in donor assistance to fight malaria.
Between 2002 and 2007 we estimate that a minimum annual average of approximately US$1 billion is committed by the international financing agencies and domestic sources across 87 PfMECs. In these countries 2.37 billion people are at risk of infection, including 1.4 billion people who live in areas where the risks of infection are classified as stable and likely to bear the highest clinical and economic burdens (Table 1). The most important contributor to malaria financing is the GFATM. In the 6 y since the inception of the GFATM, over US$2.4 billion has been approved for funding across 76 PfMECs, 41% of the estimated combined annual support to the P. falciparum world. Of the approved funding, most (78%) was targeted at the continent with the highest concentration of people living under conditions of stable transmission and representing the poorest countries globally: Africa (Table 1). Africa also received more than half (59%) of the audited non-GFATM financing, including notable awards made by the bilateral agencies of the US and the UK, the World Bank global strategy and booster program, and PMI.
Given the scarcity of domestic resources and the greatest disease burden across much of the African continent, this allocation of global malaria financing seems appropriate, if taken at face value, but in fact it might not be adequate. Funding commitments by the GFATM and other sources also appear to be distributed appropriately among the lowest population at risk areas of the Americas/Caribbean and Mid East/East Europe (Table 1). However, the South East Asia/Western Pacific region of the world is home to 47% of the global population at risk of stable P. falciparum transmission, yet these countries were awarded only 17% of the GFATM funding between 2002 and 2007 and 24% of the non-GFATM support, representing a combined annualized per capita at stable risk commitment of approximately US$1 (Table 1). India, Indonesia, and Myanmar combined have approximately 526 million people living under conditions of stable P. falciparum infection risk, or 38% of the global PfPARstable. Yet India has been approved the equivalent of US$0.03 per capita-at-risk of stable malaria p.a. by the GFATM, and its alternative funding sources amount to only US$0.20 per capita-at-risk p.a. (Table S1). GFATM support for Indonesia and Myanmar was equivalent to only US$0.12 and US$0.01 per capita-at-risk p.a., respectively. These are clear examples of the importance of examining financial planning and commitments against objectively mapped criteria of malaria risk. If development goals have a global horizon, reducing the worldwide burden of malaria by 50% by 2015 [10] would fail by a large margin unless international support were directed to those few countries likely to harbour the highest populations at risk.
Identifying Financial Need for Malaria Control at the National Level
Country-level examinations of the data revealed that 11 countries have not received any GFATM support but represented extremely low (n = 7) or zero (n = 3) populations exposed to stable P. falciparum risk and/or had a high domestic support or capacity for malaria control (e.g., Saudi Arabia and Brazil; Table S1). Conversely, the per capita under-funding of India, Indonesia, and Myanmar has been described, but two additional countries should be highlighted for which a low funding commitment is evident when computed as per capita-at-risk. Nigeria, the second-largest PfPARstable country in the world with almost 135 million people at risk, was awarded only US$0.12 per capita-at-risk p.a. by the GFATM between 2002 and 2007 (20% of all enumerated per capita investment). Pakistan, with 31 million people at risk, was awarded only US$0.1 per capita p.a. support by the GFATM and has very little domestic or other external support (approximately US$0.016 per capita p.a.).
The example of Suriname shows how much funding can be committed by the GFATM to a comparatively small population at risk and with an existing substantial financial non-GFATM support. Here we found that the GFTAM had approved US$147 per capita at stable risk p.a. and the combined commitments to malaria amounted to US$167 per capita p.a. Suriname represents an outlier in the analysis and is expected to achieve rapid scale-up results toward achieving its malaria MDG [55].
We have not analysed the performance consequences of increased international support to malaria since 2002. Analysis of commodity procurements through GFATM has been used to support the successes of the mechanism in reaching international coverage targets rather than actual data on intervention coverage or health impact [4,56]. Claims of performance success by the World Bank [57] have been criticised, as they were not based on validated health impact data [58]. The results published recently by UNICEF and the RBM partnership [59] suggest that progress is being made in some countries, and this progress is assumed to be a direct result of increased availability of funding. There has not been a detailed comparison of performance targets (judged by intervention coverage or health impact) against a per capita financial commitment among populations at risk of P. falciparum malaria. Such an analysis will only be possible when a temporally congruent series of country-by-country survey data is assembled. To date only 16 PfMECs have national survey data on malaria intervention coverage after 2005 [59].
Auditing international health financing is difficult [36], and our analysis and accompanying data (Table S1) come with additional caveats. The GFATM is transparent in the financial information they provide on funding and is a model for all donors. The precise contribution of funding for malaria control to specific countries by the bilateral donor agencies between 2002 and 2007 is harder to define. First, with the recent exception of USAID and DFID, bilateral agencies make little attempt to provide disease-specific, country-specific accounts. Second, for all development agencies, malaria support is hard to separate from general health sector support or direct-budgetary support that assists human resources, essential drugs, and infrastructure. These contributions are substantial in many resource-poor countries, but there is no obvious method to segregate the malaria-specific component. Estimating domestic expenditure on national malaria control is also difficult to determine. National governments support their health systems to deliver goods and services, and in many African countries the single largest disease burden on the health system is malaria. We have defaulted to using data provided by the WHO on domestic funding for malaria [32] and have assumed that these data include a national government's audited budgetary allocation under a specified line item labelled “malaria.” Precisely how these figures were presented to WHO remain unclear, for example, whether the reported figures included funds derived from direct budgetary support from bilateral agencies or not. The fidelity of the information is questionable. Malawi was reported to spend US$22.2 million p.a., a country five times smaller than Kenya, which reported only US$0.082 million government commitment, which in turn was half the value supposedly committed by its neighbour, Somalia (US$0.16 million), during a period when the central government in Somalia was not operational.
Defining the financial commitments at country levels, from either provider or recipient sources, limits the overall capacity of financial needs assessments on a country-by-country basis. Increasing pressure on international donors to improve the audited country-by-country transparency in financial commitments will make the sorts of equity analysis presented here possible for all funding partners. National governments, as partners in the RBM partnership, should be encouraged to and assisted in developing better metrics to enumerate annual financial flows to malaria control. RBM is currently undertaking a needs-assessment exercise in 25 African countries, and this should improve the data currently assembled in Table S1.
The Adequacy of the Global Financial Commitment to Malaria Control
Most methods of estimating financial need assume a similar costing strategy and package of effective interventions reaching scales of 80% coverage: insecticide-treated nets; indoor-residual house spraying; prevention of malaria in pregnancy; prevention and control of epidemics; effective case management with artemisinin-based combination therapy combined with improved diagnostics; management of severe malaria; and supporting structures such as communication, training, surveillance, and monitoring. The outcomes of these modelled assumptions of need vary considerably globally and regionally, largely as a result of variations in methods used to estimate the denominators of populations at risk. The earliest attempts by Narasimhan and Attaran [36] estimated that global needs ranged between US$1.5 billion and US$2.5 billion p.a. Recent estimates doubled these figures to between US$3.8 billion and US$4.5 billion p.a. globally with per capita needs for Africa of US$2.43, for Asia and Oceania of US$1.16, and for the Americas of US$0.86 in 2006 [33]. Using a different denominator, Teklehaimanot et al. [60] estimated that the per capita needs for effective scaling up of malaria control was US$4.46 in Africa in 2007, or a combined commitment from all sources of approximately US$3 billion.
Using the range of predicted annual needs we estimate that there remains a 50%–450% shortfall of funding to achieve the scaling up of malaria control required worldwide. It should be noted that these estimates are only for the scaling up of control and do not even consider the additional financing that might be required for malaria elimination, where feasible. This shortfall is particularly acute in several high population density countries with stable P. falciparum malaria risk. In Africa, where it will be difficult to increase domestic financing appreciably before 2015, there is still a possible 80%–90% deficit in per capita funding for effective malaria control.
Analyses, Caveats, and Future Perspectives
There are important epidemiological heterogeneities within our definition of stable malaria, ranging from less than one infectious bite from local dominant vectors per person per year to over ten new infections per person per night. The prevalence of infection in a community is a useful guide to the variation in intensity of transmission within areas of stable endemicity [14,61,62]. In addition, it was recently demonstrated that the prevalence of infection is lower across the stable endemic areas of the Americas, much of Asia, and a larger part of Africa than was previously assumed [13]. The public health consequences of P. falciparum in areas of low transmission intensity will be considerably less than those in areas of more intense transmission [22,63]. However, even in areas of high transmission the relationship between disease and its outcome is nonlinear and complex [22,63,64]. We have not adjusted our analysis of funding allocation according to the intensity of transmission within and between the stable P. falciparum–endemic countries for three reasons. First, there is no adequate description of transmission intensity across the World that is related to parameters that can be used to define the public health burdens at country levels. Second, the GFATM and RBM do not currently have a working model or definition of the projected public health benefits resulting from financing different intervention mixes of known efficacy under different starting transmission conditions. Hence, the GFATM uses overall populations at risk rather than transmission intensity, vector species, or other proximate determinants of infection risk as its guiding mantra for resource allocation [1,6]. Third, we have assumed that a standard mix of interventions would be required to meet the objectives of RBM that were laid out in the late 1990s.
As the malaria control community and many individual countries reconsider prospects for the elimination of malaria where feasible, resource needs and matched financial expectations may change. It is not clear whether the financial needs of a country predominantly exposed to intense, stable P. falciparum malaria transmission are greater than those of a country whose dominant transmission characteristics are of low intensity. The interventions and their delivery mechanisms may become more expensive as countries prepare to target focal pockets of infection risk and prevent the re-entry of transmission into malaria-free areas. The use of stable, endemic, high disease–burden definitions of populations at risk may change, and it may become more “acceptable” to consider unstable transmission–risk populations as a target for funding. We believe that the uncertainty around priority setting based on risk, intervention selection, and the consequent planning of financial needs requires a clear approach by the GFATM, RBM, and their partners, informed by the baseline endemicity of malaria within the stable limits of P. falciparum malaria transmission.
With these caveats our analysis has focussed on appropriate allocations based on overall PfPAR, but it has not examined the adequacy of funding. Nor have we considered the political dimensions of international health financing, for example, whether donor agencies should provide external support to countries with corrupt administrations or to those whose national governments spend much less on public health than on military expansion. These questions are beyond the scope of the present analysis, but we hope that the biological risk and country-level funding data in Table S1 offer a combined basis for examining other dimensions and nuances of malaria financing.
Conclusion
That more funding is needed to control P. falciparum malaria is not a new concept, but our analysis highlights the reality that more is needed in specific countries and regions to ensure that the highest concentrations of people at risk benefit from international support. Without a selective epidemiological–economic targeting of global malaria control investment it seems unlikely that international goals to halve disease burdens by 2015 will be achieved.
Supporting Information
Zdroje
1. GFATM
2007
The framework document of the Global Fund to Fight AIDS, Tuberculosis and Malaria. Title, purpose, principles and scope of the fund
Geneva
GFTAM
Available: http://www.theglobalfund.org/en/files/publicdoc/Framework_uk.pdf. Accessed 28 January 2008.
2. GFTAM
2007
The Global Fund. Who we are what we do
Geneva
GFTAM
Available: http://www.theglobalfund.org/en/media_center/publications/brochure/. Accessed 28 January 2008.
3. GFTAM
2008
Investing in our future.
The Global Fund to Fight AIDS, Tuberculosis and Malaria
Geneva
GFTAM
Available: http://www.theglobalfund.org/. Accessed: 28 January 2008.
4. FeachemRGASabotOJ
2006
An examination of the Global Fund at 5 years.
Lancet
368
537
540
5. NahlenBLLow-BeerD
2007
Building to collective impact: the global fund support for measuring reduction in the burden of malaria.
Am J Trop Med Hyg
77
321
327
6. GFTAM
2007
Resource needs: funding the global fight against HIV/AIDS, tuberculosis and malaria
Geneva
GFTAM
Available: http://www.theglobalfund.org/en/files/about/replenishment/assessment_report_en.pdf. Accessed: 28 January 2008.
7. GFATM
2006
Report of the Policy and Strategy Committee
Thirteenth board meeting,
26–27 April 2006.
Geneva
GFTAM
Available: http://www.theglobalfund.org/en/files/boardmeeting13/GF-B13–7_Report_of_the_Policy_and_Strategy_Commmitee.pdf. Accessed: 28 January 2008.
8. World Bank
2007
The World Bank global strategy and booster program
Washington (D. C.)
World Bank
Available: http://web.worldbank.org/WBSITE/EXTERNAL/TOPICS/EXTHEALTHNUTRITIONANDPOPULATION/EXTMALARIA/0,,contentMDK:20461038~pagePK:210058∼piPK:210062~theSitePK:377598,00.html. Accessed: 28 January 2008.
9. PMI
2007
President's Malaria Initiative. Saving the lives of mothers and children in Africa. First annual report, March 2007
Washington (D. C.)
President's Malaria Initiative (PMI)
Available: http://www.fightingmalaria.gov/resources/pmi_annual_report.pdf. Accessed: 28 January 2008.
10. MDG
2008
UN Millennium Development Goals
New York
United Nations
Available: http://www.un.org/millenniumgoals/. Accessed: 28 January 2008.
11. SachsJD
2004
Health in the developing world: achieving the Millennium Development Goals.
Bull World Health Organ
82
947
949
12. HaySISnowRW
2006
The Malaria Atlas Project: developing global maps of malaria risk.
PLoS Med
3
e473
doi:10.1371/journal.pmed.0030473.
13. GuerraCAGikandiPWTatemAJNoorAMSmithDL
2008
The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide.
PLoS Med
5
e38
doi:10.1371/journal.pmed.0050038.
14. HaySISmithDLSnowRW
2008
Measuring malaria endemicity from intense to interrupted transmission.
Lancet Inf Dis
8
369
378
15. PampanaE
1969
A textbook of malaria eradication
2nd edition.
London
Oxford University Press
593
16. SwaroopSGilroyABUemuraK
1966
Statistical methods in malaria eradication.
World Health Organization Monograph Series, no. 51
Geneva
World Health Organization
164
17. YekutielP
1960
Problems of epidemiology in malaria eradication.
Bull World Health Organ
22
669
683
18. YekutielP
1980
The global malaria eradication campaign.
In:
KlingbergMA
Eradication of infectious diseases: a critical study. 2. Contributions to epidemiology and biostatistics
Basel (Switzerland)
Karger
34
88
19. BalkDLDeichmannUYetmanGPozziFHaySI
2006
Determining global population distribution: methods, applications and data.
Adv Parasitol
62
119
156
20. UNPOP
2006
World population prospects: the 2006 revision population database
New York
United Nations Population Division (UNPOP)
Available: http://esa.un.org/unpp/. Accessed: 28 January 2008.
21. HaySINoorAMNelsonATatemAJ
2005
The accuracy of human population maps for public health application.
Trop Med Int Health
10
1073
1086
22. SnowRWGuerraCANoorAMMyintHYHaySI
2005
The global distribution of clinical episodes of Plasmodium falciparum malaria.
Nature
434
214
217
23. RBM
2002
The Abuja declaration and the plan of action. An extract from the African Summit on Roll Back Malaria, Abuja, 25 April 2000. WHO/CDS/RBM/2000.17
Geneva
RBM
Available: http://www.rollbackmalaria.org/docs/abuja_declaration.pdf. Accessed 28 January 2008.
24. RBM
2008
The Roll Back Malaria partnership
Geneva
Roll Back Malaria
Available: http://www.rbm.who.int/. Accessed: 28 January 2008.
25. RosenbergR
2007
Plasmodium vivax in Africa: hidden in plain sight.
Trends Parasitol
23
193
196
26. SattabongkotJTsuboiTZollnerGESirichaisinthopJCuiLW
2004
Plasmodium vivax transmission: chances for control.
Trends Parasitol
20
192
198
27. MayxayMPukrittayakameeSNewtonPNWhiteNJ
2004
Mixed-species malaria infections in humans.
Trends Parasitol
20
233
240
28. BairdJK
2007
Neglect of Plasmodium vivax malaria.
Trends Parasitol
23
533
539
29. PriceRNTjitraEGuerraCAWhiteNJAnsteyNM
2007
Vivax malaria: neglected and not benign.
Am J Trop Med Hyg
77
79
87
30. LuCMichaudCMKhanKMurrayCJL
2006
Absorptive capacity and disbursements by the Global Fund to Fight AIDS, Tuberculosis and Malaria: analysis of grant implementation.
Lancet
368
483
488
31. TeklehaimanotASnowRW
2002
Will the Global Fund help roll back malaria in Africa.
Lancet
360
888
889
32. WHO
2005
World Malaria Report 2005
Geneva
World Health Organization (WHO)
Available: http://www.rbm.who.int/wmr2005/. Accessed: 28 January 2008.
33. KiszewskiAJohnsBSchapiraADelacolletteCCrowellV
2007
Estimated global resources needed to attain international malaria control goals.
Bull World Health Organ
85
623
630
34. Department for International Development
2007
Malaria fact sheet
London
DFID
Available: http://www.dfid.gov.uk/pubs/files/mdg-factsheets/malariafactsheet.pdf. Accessed: 28 January 2008.
35. USAID
2008
USAID from the American people
Washington (D. C.)
United States Agency for International Development (USAID)
Available: http://www.usaid.gov/. Accessed: 28 January 2008.
36. NarasimhanVAttaranA
2003
Roll back malaria? The scarcity of international aid for malaria control.
Malar J
2
8
37. Malaria No More
2008
Malaria No More.
Take the giving challenge
New York
Malaria No More
Available: http://www.malarianomore.org. Accessed: 28 January 2008.
38. UNITAID
2008
UNITAID. Making globalization equitable
Geneva
UNITAID
Available: http://www.unitaid.eu/how-it-is-financed.html. Accessed: 28 January 2008.
39. World Swim against Malaria
2008
Welcome to World Swim against Malaria
London
World Swim against Malaria
Available: http://www.worldswimagainstmalaria.com. Accessed: 28 January 2008.
40. Clinton Foundation
2008
William J. Clinton Foundation
New York
Clinton Foundation
Available: http://www.clintonfoundation.org. Accessed: 28 January 2008.
41. Médecins sans Frontières
2008
Médecins sans Frontières
Geneva
Médecins sans Frontières (MSF)
Available: http://www.msf.org. Accessed: 28 January 2008.
42. African Development Bank Group
2008
African Development Bank Group. Building today, a better Africa tomorrow
Abidjan
African Development Bank Group
Available: http://www.afdb.org. Accessed: 28 January 2008.
43. Asian Development Bank
2008
Asian Development Bank.
Fighting povert in Asia and the Pacific
Metro Manila
Asian Development Bank
Available: http://www.adb.org. Accessed: 28 January 2008.
44. Islamic Development Bank
2008
Islamic Development Bank.
Together we build a better future
Jeddah
Islamic Development Bank
Available: http://www.isdb.org. Accessed: 28 January 2008.
45. African Medical Research Foundation
2008
African Medical Research Foundation
Nairobi
African Medical Research Foundation
Available: http://www.amref.org. Accessed: 28 January 2008.
46. Merlin
2008
Merlin. Medical relief, lasting care
London
Merlin
Available: http://www.merlin.org.uk. Accessed: 28 January 2008.
47. World Vision International
2008
World Vision International: World Vision International
Available: http://www.wvi.org. Accessed: 28 January 2008.
48. PATH
2008
New partnership launched to accelerate and evaluate national malaria control program in Africa
Seattle
PATH
Available: http://www.path.org/news/pr-050519-macepa.php. Accessed: 28 January 2008.
49. MeleigyM
2007
Arabian Peninsula states launch plan to eradicate malaria.
BMJ
334
117
50. RoweAKRoweSYSnowRWKorenrompELSchellenbergJRA
2006
The burden of malaria mortality among African children in the year 2000.
Int J Epidemiol
35
691
704
51. SnowRWCraigMDeichmannUMarshK
1999
Estimating mortality, morbidity and disability due to malaria among Africa's non-pregnant population.
Bull World Health Organ
77
624
640
52. RobertsLEnserinkM
2007
Did they really say … eradication.
Science
318
1544
1545
53. FeachemRSabotO
2008
A new global malaria eradication strategy.
Lancet
10
1633
1635
54. NabarroDNTaylerEM
1998
The “Roll Back Malaria” campaign.
Science
280
2067
2068
55. SmithC
2007
Suriname has ‘already hit’ malaria MDG
London
Science and Development Network
Available: http://www.scidev.net/content/news/eng/suriname-has-already-hit-malaria-mdg.cfm. Accessed: 28 January 2008.
56. KomatsuRLow-BeerDSchwartlanderB
2007
Global Fund-supported programmes contribution to international targets and the Millennium Development Goals: an initial analysis.
Bull World Health Organ
85
805
811
57. BaratLM
2006
Four malaria success stories: how malaria burden was successfully reduced in Brazil, Eritrea, India, and Vietnam.
Am J Trop Med Hyg
74
12
16
58. BateR
2008
World Bank matrix malaria booster program an analysis
Washington (D. C.)
Africa Fighting Malaria
Available: http://www.fightingmalaria.org/pdfs/WB_matrixcomment.pdf. Accessed: 28 January 2008.
59. UNICEF
2007
Malaria and Children. Progress in Intervention Coverage
New York
The United Nations Children's Fund (UNICEF)
Available: http://www.unicef.org/health/files/MalariaOct6forweb_final.pdf. Accessed: 28 January 2008.
60. TeklehaimanotAMcCordGCSachsJD
2007
Scaling up malaria control in Africa: an economic and epidemiological assessment.
Am J Trop Med Hyg
77
138
144
61. SmithDLMcKenzieFE
2004
Statics and dynamics of malaria infection in Anopheles mosquitoes.
Malar J
3
13
62. SmithDLDushoffJSnowRWHaySI
2005
The entomological inoculation rate and Plasmodium falciparum infection in African children.
Nature
438
492
495
63. SnowRWMarshK
2002
The consequences of reducing transmission of Plasmodium falciparum in Africa.
Adv Parasitol
52
235
264
64. TrapeJFRogierC
1996
Combating malaria morbidity and mortality by reducing transmission.
Parasitol Today
12
236
240
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2008 Číslo 7- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Metabolit živočišné stravy produkovaný střevní mikroflórou zvyšuje riziko závažných kardiovaskulárních příhod
-
Všetky články tohto čísla
- International Funding for Malaria Control in Relation to Populations at Risk of Stable Transmission
- Immunological Outcomes of New Tuberculosis Vaccine Trials: WHO Panel Recommendations
- A 58-Year-Old Woman with Abdominal Symptoms and Elevated C-Reactive Protein
- Organophosphate Poisoning–Induced Intermediate Syndrome: Can Electrophysiological Changes Help Predict Outcome?
- Evidence-Based Tuberculosis Diagnosis
- Divergent Goals and Commitments in Global Malaria Intervention
- Next Stop, Don't Block the Doors: Opening Up Access to Clinical Trials Results
- The Effects of International Monetary Fund Loans on Health Outcomes
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Evidence-Based Tuberculosis Diagnosis
- Organophosphate Poisoning–Induced Intermediate Syndrome: Can Electrophysiological Changes Help Predict Outcome?
- The Effects of International Monetary Fund Loans on Health Outcomes
- Next Stop, Don't Block the Doors: Opening Up Access to Clinical Trials Results
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



