-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Immunohistochemical analysis of CD9, CD29 and epithelial to mesenchymal transition in triple-negative breast cancer
Imunohistochemická analýza CD9, CD29 a epitelo-mezenchymové tranzice u triple-negativního karcinomu prsu
Východiska: Triple-negativní karcinomy prsu (TNBC) jsou heterogenní skupinou nádorů s převážně agresivním chováním a špatnou prognózou. V souvislosti s jejich agresivním chováním a chemorezistencí vůči léčbě se do popředí dostal koncept epitelo-mezenchymové tranzice (EMT). Proteiny CD9 a CD29 jsou spojeny s EMT a mohou hrát roli v progresi TNBC. Naším cílem bylo prozkoumat asociaci těchto markerů s metastázami do lymfatických uzlin, gradingem tumoru, proliferační aktivitou a přežitím pacientů. Pacienti a metody: Náš soubor tvořilo 66 pacientek s TNBC bez neoadjuvantní terapie ve věku 26–81 let. Patologické stadium nádoru se pohybovalo od pT1b do pT3 a histologický stupeň od II do III podle systému Bloom-Richardson. Imunohistochemické hodnocení exprese CD9, CD29, E-cadherinu, vimentinu, androgenového receptoru a Ki-67 bylo provedeno semikvantitativně pomocí H-skóre. Exprese proteinů byla statisticky hodnocena ve vztahu ke klinicko-patologickým parametrům a přežití pacientů. Výsledky: Pozorovali jsme nižší expresi CD9 v metastázách lymfatických uzlin ve srovnání s primárním nádorem (p = 0,021). Exprese CD29 v primárním nádoru byla signifikantně nižší u pacientů s metastázami v lymfatických uzlinách ve srovnání s pacienty bez diseminace (p = 0,03). Ani exprese CD9 ani CD29 proteinu nebyla spojena s přežitím specifickým pro karcinom prsu (BCSS). Nižší exprese E-cadherinu na periferii primárního tumoru byla spojena s horším BCSS (p = 0,038). Pro grading ani přítomnost metastáz v lymfatických uzlinách nebyl nalezen signifikantní vztah s BCSS. Nižší exprese E-cadherinu na periferii byla také spojena s vyšší hladinou Ki67 (Rs −0,26) a vimentinu (Rs −0,33). Závěr: Snížená exprese proteinů CD9 a CD29 byla spojena s růstem metastáz v lymfatických uzlinách, avšak jejich souvislost s přežitím nebyla prokázána. Nižší exprese E-cadherinu na periferii primárního nádoru byla spojena s vysokou proliferací a špatným nádorově specifickým přežitím.
Klíčová slova:
triple-negativní karcinom prsu – CD9 – CD29 – E-cadherin – epitelo-mezenchymová tranzice
Authors: R.- Ondruššek 1 3; S. Brychtová 1; M. Bezděková 1; K. Bouchalova 4; Z. Vávrová 5; K. Souček 6; J. Bouchal 1
Authors place of work: Department of Clinical and Molecular Pathology, Palacký University and University Hospital Olomouc, Czech Republic 1; EUC Laboratoře CGB a. s. Ostrava, Czech Republic 2; Department of Molecular and Clinical Pathology and Medical Genetics, University Hospital Ostrava, Czech Republic. 3; Department of Pediatrics, Palacký University and University Hospital Olomouc, Czech Republic 4; Department of Surgery, AGEL Hospital Ostrava-Vítkovice, Czech Republic 5; Department of Cytokinetics, Institute of Biophysics of the Czech Academy of Sciences, Brno, Czech Republic, † Deceased 6
Published in the journal: Klin Onkol 2024; 37(1): 50-56
Category: Původní práce
doi: https://doi.org/10.48095/ccko202450Summary
Background: Triple-negative breast carcinomas (TNBC) are a heterogeneous group of tumors with mostly aggressive behaviour and poor prognosis. In association with their aggressive behavior and chemoresistance to treatment, the concept of epithelial-mesenchymal transition (EMT) has come to the fore. CD9 and CD29 proteins are associated with EMT and may play a role in TNBC progression. Our aim was to investigate association of these markers with the lymph node metastasis, tumor grade, proliferative activity, and patient survival. Patients and methods: Our cohort consisted of 66 TNBC patients without neoadjuvant therapy, aged 26–81 years. The pathological tumor stages ranged from pT1b to pT3 and histological grades ranged from II to III, according to the Bloom-Richardson system. Immunohistochemical evaluation of CD9, CD29, E-cadherin, vimentin, androgen receptor and Ki-67 expression was performed semiquantitatively using the H-score. Expression of the proteins was statistically evaluated in relation to the clinicopathological parameters and survival of the patients. Results: We observed lower expression of CD9 in lymph node metastases compared to the primary tumor (P = 0.021). The CD29 expression in primary tumor was significantly lower in patients with lymph node metastases compared to patients without cancer dissemination (P = 0.03). Neither CD9 nor CD29 protein expression was associated with breast cancer-specific survival (BCSS). Lower expression of E-cadherin at the periphery of the primary tumor was associated with worse BCSS (P = 0.038). Neither grade nor the presence of lymph node metastases reached significant association with the BCSS. Lower expression of E-cadherin at the periphery was also associated with higher Ki67 (Rs −0.26) and vimentin (Rs −0.33). Conclusion: Decreased protein expression of CD9 and CD29 were associated with lymph node metastasis growth, however, their association with survival was not proved. Lower expression of E-cadherin at the periphery of the primary tumor was associated with high proliferation and poor breast cancer-specific survival.
Keywords:
epithelial-mesenchymal transition – triple-negative breast cancer – CD9 – CD29 – E-cadherin
Introduction
Mammary carcinomas stand out as the most prevalent malignancy affecting women, constituting approximately 24% of all malignancies globally [1]. Among these, triple-negative breast cancers (TNBC) form a highly diverse group of tumors, known for their aggressive nature [2]. Recurrent TNBCs result in significantly poorer long-term survival rates and an unfavorable prognosis compared to non-TNBC cases [3–6]. TNBC presents a significant clinical challenge due to its resistance to endocrine hormone therapy and other targeted treatments currently available. Ongoing research in TNBC primarily focuses on identifying novel proteins suitable for effective targeted cancer therapy [3] and discovering new prognostic markers.
One prominent exosomal marker under investigation is the tetraspanin protein CD9, which plays a crucial role in modulating cell adhesion, migration, proliferation, and vesicular transport processes, including exosomes [7,8]. CD9 plays a key role in interactions between tumor cells and the stromal microenvironment and has a major impact on tumor growth and metastasis. We have recently reviewed all immunohistochemical studies in different solid cancers; however, we concluded that CD9 is not clearly associated with either tumor suppression or promotion [9]. Additionally, CD29, also known as b1-integrin, serves as a cell surface protein receptor encoded by the ITGB1 gene, belonging to the collagen receptor family. It is commonly referred to as an epithelial-mesenchymal transition (EMT) marker [10]. CD29 regulates various biological processes, including cell proliferation, survival, and migration [11,12].
EMT, the epithelial-mesenchymal transition, is a phenomenon closely associated with malignant tumor progression and metastasis [13]. It enables a polarized epithelial cell, typically anchored to the basement membrane, to undergo biochemical changes, leading to the adoption of a mesenchymal cell phenotype. This transformation includes heightened migratory capacity, invasiveness, increased resistance to apoptosis, and significantly augmented production of extracellular matrix components, collectively forming the tumor microenvironment [13–15]. EMT frequently accompanies the progression of TNBC and contributes to its resistance to cancer therapy [2].
We have previously found by flow cytometry a decreased CD9 and CD29 expression in breast cancer cells that underwent EMT [16]. We now aimed to investigate CD9 and CD29 expression along with E-cadherin and vimentin (EMT markers) in a cohort of 66 TNBC patients without neoadjuvant therapy. The formalin-fixed paraffin-embedded (FFPE) samples from primary tumors were carefully selected and lymph node metastases were also included from 17 patients. Importantly, information on the clinical follow-up and survival of the patients was chased up.
Tab. 1. Clinicopathological features of triple negative breast cancer cohort (N = 66). 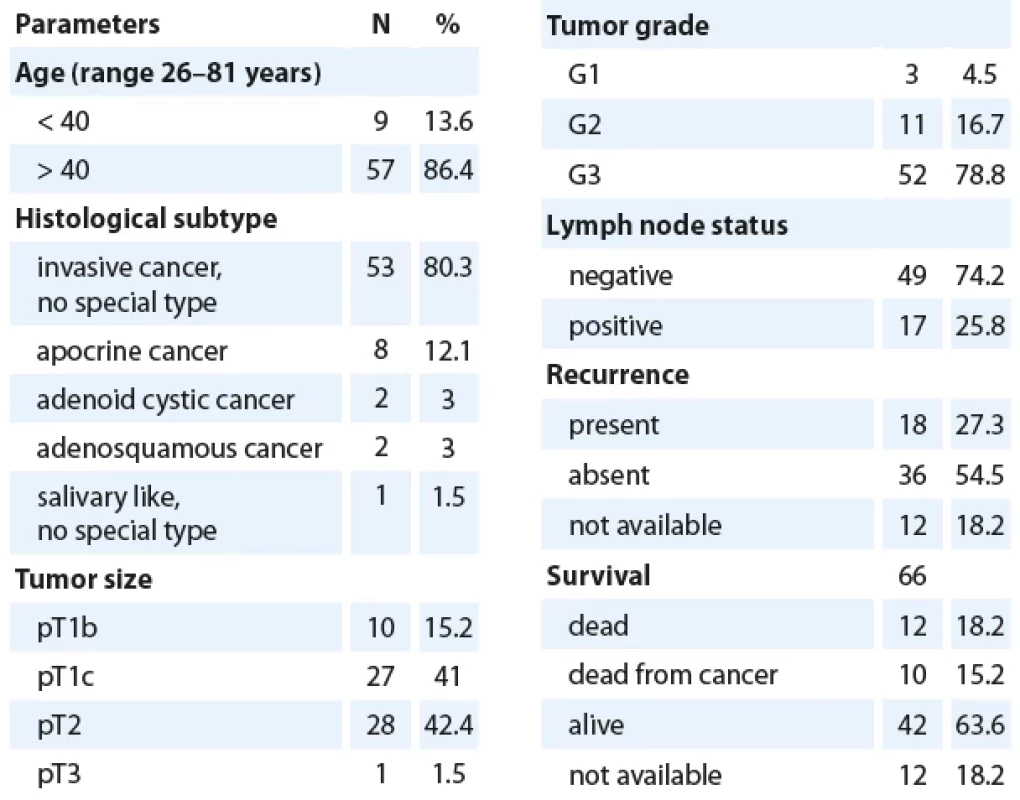
Patients and methods
Patients characteristics
Our cohort (Tab. 1) consisted of 66 patients with triple-negative breast cancer diagnosed from biopsy of surgical specimens of breast (quadrantectomy or mastectomy samples from University Hospital Ostrava, AGEL Hospital Ostrava-Vítkovice, Hospital Karviná-Ráj and EUC Klinika Kladno), which were examined during the years 2013–2022. The patients‘ age ranged from 26 to 81 years, with pathological tumor stages ranging from pT1b to pT3 and histological grades ranging from II to III according to the Bloom-Richardson system. Patients who received neoadjuvant therapy were excluded from the study. TNBC was defined as carcinomas showing simultaneous immunohistochemical negativity for estrogen and progesterone receptors, Her2/ neu, and confirmed negativity through genetic fluorescent in situ hybridization (FISH). The series included 53 tumors with invasive carcinoma of the NST type, 8 apocrine carcinomas, 2 adenoid cystic carcinomas, 2 adenosquamous carcinomas, and 1 “salivary-like” carcinoma without further specification.
Immunohistochemistry
Tissue samples were fixed in 10% formalin and embedded in paraffin, then they were cut into 2–3-μm sections. Selected proteins were investigated by indirect immunohistochemistry using specific monoclonal antibodies: rabbit anti-CD9 (clone EPR 2949, diluted 1 : 2,000; Abcam), monoclonal rabbit CD29 antibodies (clone EP1041Y, diluted 1 : 2,000; Abcam), mouse anti-E-cadherin (clone NCH-38, diluted 1 : 50, Dako), mouse anti-vimentin (clone V9, diluted 1 : 100, Dako), and anti-Ki67 (clone 30-9, Ventana).
The immunohistochemical assessment of CD9 and CD29 expression was performed semiquantitatively using the H (histo) score, which included percentage of positivity and a 4-level grading of staining intensity: 0 – no expression, 1 – low intensity, 2 – moderate intensity, 3 – strong intensity. Protein expression was monitored in the tumor center and its periphery, both in tumor cells and stroma, simultaneously with its presence in lymphocytes and further in the surrounding non-tumor breast tissue.
Fig. 1. An example of high expression of CD9 in the primary tumor and low level in the lymph node metastasis from the same patient. 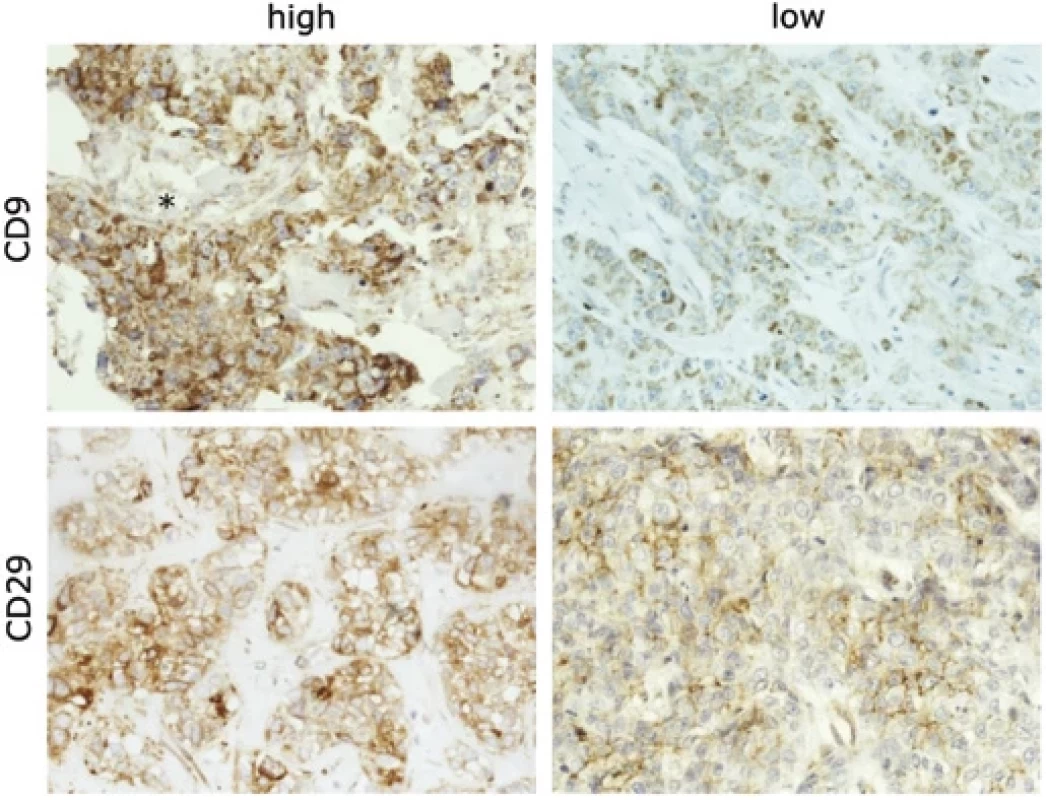
Extracellular positivity of CD9 was occasionally observed (indicated by asterisk). The lower panel shows an example of high CD29 expression in a primary tumor without dissemination and low expression of CD29 in a patient with lymph node metastasis. Magnification 100×. Statistical analysis
The results were statistically evaluated using the Mann-Whitney U test, the Wilcoxon test, and the Spearman‘s rank correlation coefficient along with the Kaplan-Meier survival analysis with the log-rank test (STATISTICA 12, TIBCO Software).
Results
An immunohistochemical study was conducted on a cohort of 66 TNBC patients without neoadjuvant therapy (Tab. 1). Most patients with high-grade tumors received adjuvant chemotherapy with anthracycline-cyclophosphamide and taxanes. One patient was treated with carboplatin and gemcitabine, and two patients received capecitabine. Information on the clinical follow-up and survival of the patients was chased up. Breast cancer specific survival (BCSS) was defined as the time from diagnosis to death from breast cancer, while overall survival (OS) was related to death from any cause.
We found a significant decrease in CD9 expression in nodal metastases in comparison to primary carcinomas (P = 0.021) (Fig. 1, Graph 1A). Interestingly, we also observed an extracellular CD9 positivity (Fig. 1) which was manifested in majority of grade 3 tumors (16 out of 17 cases); however, it did not associate with nodal positivity or higher pT stages. Expression of CD9 was also found in lymphocytes, which was significantly higher in grade 3 tumors in comparison with grades 1 and 2 (P = 0.011). As expected, strong association with a high grade was observed for a high Ki67 proliferation marker (P < 0.001).
The CD29 marker demonstrated both cytoplasmic and membrane expression. The predominant membrane expression of CD29 in tumor cells was significantly lower in carcinomas forming lymphatic metastases (P = 0.030) compared to localized pTN0 tumors (Fig. 1 and Graph 1B). Neither CD29 nor CD9 expression was associated with survival. Importantly, reduced E-cadherin expression at the periphery of primary tumors correlated with poor BCSS (P = 0.038) (Graph 1C and Fig. 2). Moreover, we established associations between E-cadherin, vimentin, and proliferation marker Ki67. Diminished E-cadherin expression at the periphery correlated with higher Ki67 (Rs −0.26) and vimentin (Rs −0.33) levels (Fig. 2). The most important results are also summarized in Tab. 2.
Graph 1. Relationship of CD9, CD29 and E-cadherin to lymph node metastasis and breast cancer specific survival (BCSS). 
The expression of CD9 was significantly higher in primary tumors compared to lymph node metastases (P = 0.021). B) The expression of CD29 was significantly higher in patients with localized disease compared to tumors with lymph node metastases (P = 0.030). C) The lower expression of E-cadherin at the periphery of the primary tumor was associated with worse BCSS (P = 0.038). The box-plots represent 25–75 percentiles, median and range of values. LN – lymph nodes Fig. 2. An example of triple negative breast cancer. 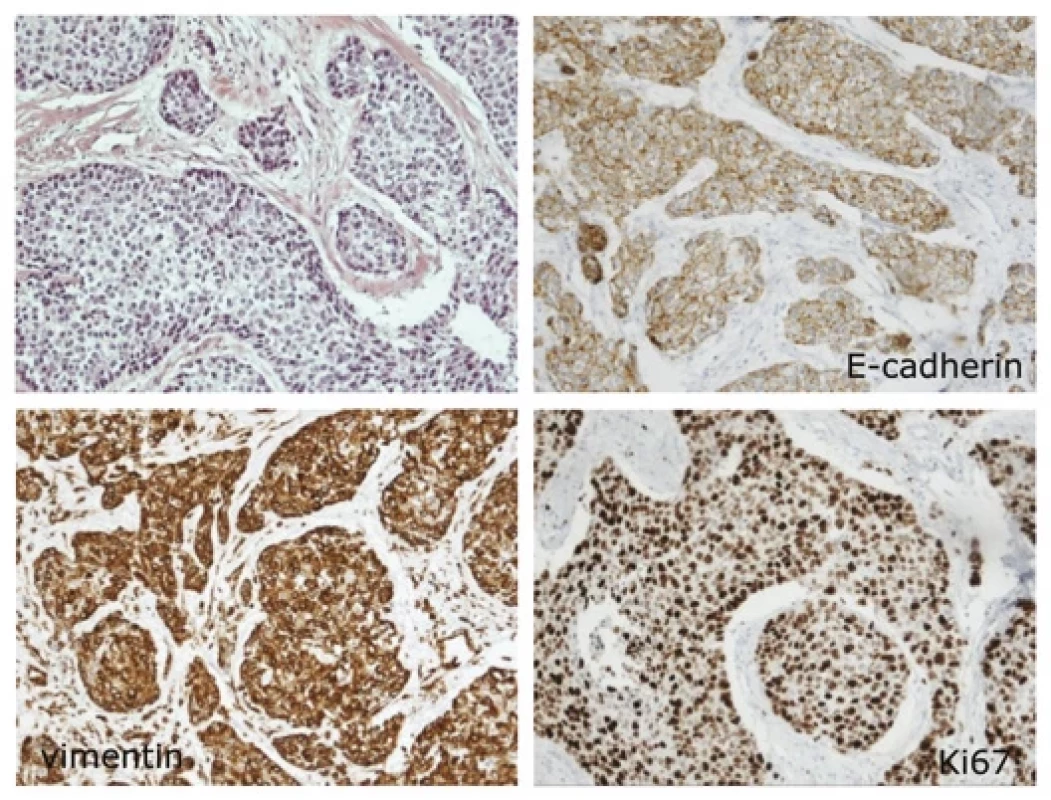
The tumor consists of solid groups of cells and displays low expression of E-cadherin at the periphery of the tumor and high expression of vimentin and Ki67. Magnification 100× (for hematoxylin-eosin staining and for immunohistochemical staining of proteins). Tab. 2. Major findings of the study. 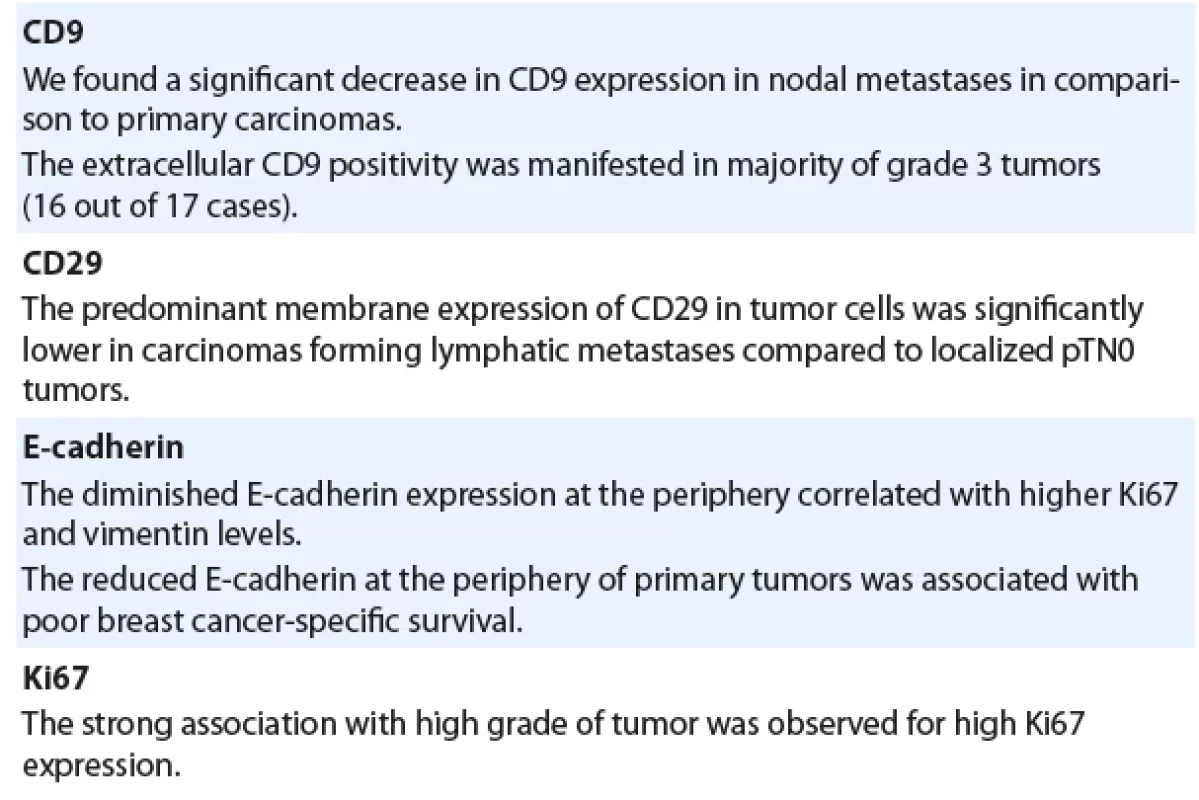
Discussion
Our study unveiled a diminished expression of CD9 in nodal metastases, consistent with existing literature [17,18]. CD9 has been extensively studied as a prognostic marker for solid tumors. Majority of studies indicate a worse prognosis for CD9-low tumors compared to those with high expression [19–24]. However, conflicting results have also been documented [25–27]. Although we observed low expression in lymph node metastases, CD9 was not significantly associated with breast cancer specific survival.
We have also observed an extracellular CD9 positivity which may be explained by CD9 presence in membranes of exosomes, microvesicles, or apoptotic bodies [28]. Further exploration could involve alternative staining methods like TUNEL for apoptosis, or monitoring other markers of exosomes and microvesicles.
Another significant finding of our study is the association of low CD29 expression with positivity of lymph node metastases. This may agree with our previous observation of a decreased CD9 and CD29 expression in breast cancer cells that underwent EMT [16]. In this sense, loss of CD29 attenuated breast tumor growth but markedly enhanced tumor cell dissemination to the lungs [29]. These findings reveal that CD29 control a signaling network that promotes an epithelial phenotype and suppresses dissemination and indicate that targeting b1-integrins may have undesirable effects in TNBC. Still, other studies indicate worse survival of TNBC patients with high CD29 expression and targeted therapy is being tested [30,31]
The prominent marker of EMT is loss of E-cadherin which was associated with worse breast cancer specific survival in our study. Diminished E-cadherin expression at the periphery also correlated with higher Ki67 and vimentin levels. Several other studies described poor survival of TNBC patients with low E-cadherin expression [32–34] These results are also in line with our recent mass cytometry single cell analysis of fresh TNBC tissues [35] EMT score was calculated from epithelial (EpCAM + CD49f + CD9) and mesenchymal markers (vimentin + aSMA + CD44) for each cancer cell in the sample and it well associated with proliferation and lymph node colonization.
Conclusion
Decreased expression CD9 and CD29 were associated with lymph node metastasis growth, however, their association with EMT and survival was not proved. Lower expression of E-cadherin at the periphery of the primary tumor was associated with high proliferation and poor breast cancer specific survival.
Assoc. Prof. Jan Bouchal, PhD
Department of Clinical and Molecular Pathology,
Palacký University and University Hospital Olomouc
Hněvotínská 3
775 15 Olomouc
Czech Republic
e-mail: jan.bouchal@upol.czSubmitted/Obdrženo: 7. 11. 2023
Accepted/Přijato: 4. 1. 2024
Zdroje
1. Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6): 394–424. doi: 10.3322/ caac.21492.
2. Kvokačková B, Remšík J, Jolly MK et al. Phenotypic heterogeneity of triple-negative breast cancer mediated by epithelial-mesenchymal plasticity. Cancers (Basel) 2021; 13(9): 2188. doi: 10.3390/ cancers13092188.
3. Allison KH, Brogi E, Ellis IO et al. Invasive breast carcinoma: general overview. In: Allison KH, Brogi E, Ellis IO
et al. WHO Classification of Tumours. Breast tumours 5th Edition. Lyon: International Agency for Research on Cancer 2019 : 82–101.4. Bauer KR, Brown M, Cress RD et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer 2007; 109(9): 1721–1728. doi: 10.1002/ cncr.22618.
5. Blows FM, Driver KE, Schmidt MK et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med 2010; 7(5): e1000279. doi: 10.1371/ journal.pmed.1000279.
6. Liedtke C, Mazouni C, Hess KR et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 2008; 26(8): 1275–1281. doi: 10.1200/ JCO.2007.14.4147.
7. Ekström K, Crescitelli R, Pétursson HI et al. Characterization of surface markers on extracellular vesicles isolated from lymphatic exudate from patients with breast cancer. BMC Cancer 2022; 22(1): 50. doi: 10.1186/ s12885-021-08870-w.
8. Yoshioka Y, Konishi Y, Kosaka N et al. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles 2013; 2 : 20424. doi: 10.3402/ jev.v2i0.20424.
9. Ondruššek R, Kvokačková B, Kryštofová K et al. Prognostic value and multifaceted roles of tetraspanin CD9 in cancer. Front Oncol 2023; 13 : 1140738. doi: 10.3389/ fonc.2023.1140738.
10. Geng S, Guo Y, Wang Q et al. Cancer stem-like cells enriched with CD29 and CD44 markers exhibit molecular characteristics with epithelial-mesenchymal transition in squamous cell carcinoma. Arch Dermatol Res 2013; 305(1): 35–47. doi: 10.1007/ s00403-012-1260-2.
11. Clark EA, Brugge JS, Juliano RL et al. Signal transduction from the extracellular matrix. J Cell Biol 1993; 120(3): 577–585. doi: 10.1083/ jcb.120.3.577.
12. Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science 1995; 268(5208):
233–239. doi: 10.1126/ science.7716514.13. Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 2003; 112(12): 1776–1784. doi: 10.1172/ JCI20530.
14. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119(6): 1420–1428. doi: 10.1172/ JCI39104.
15. Kokkinos MI, Wafai R, Wong MK et al. Vimentin and epithelial-mesenchymal transition in human breast cancer – observations in vitro and in vivo. Cells Tissues Organs 2007; 185(1–3): 191–203. doi: 10.1159/ 000101
320.16. Remšík J, Fedr R, Navrátil J et al. Plasticity and intratumoural heterogeneity of cell surface antigen expression in breast cancer. Br J Cancer 2018; 118(6): 813–819. doi: 10.1038/ bjc.2017.497.
17. Miyake M, Nakano K, Ieki Y et al. Motility related protein 1 (MRP-1/ CD9) expression: inverse correlation with metastases in breast cancer. Cancer Res 1995; 55(18): 4127–4131.
18. Zöller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer 2009; 9(1): 40–55. doi: 10.1038/ nrc2543.
19. Arihiro K, Kaneko M, Fujii S et al. Loss of CD9 with expression of CD31 and VEGF in breast carcinoma, as predictive factors of lymph node metastasis. Breast Cancer 1998; 5(2): 131–138. doi: 10.1007/ BF02966685.
20. Houle CD, Ding X-Y, Foley JF et al. Loss of expression and altered localization of KAI1 and CD9 protein are associated with epithelial ovarian cancer progression. Gynecol Oncol 2002; 86(1): 69–78. doi: 10.1006/ gyno.2002.
6729.21. Sauer G, Windisch J, Kurzeder C et al. Progression of cervical carcinomas is associated with down-regulation of CD9 but strong local re-expression at sites of transendothelial invasion. Clin Cancer Res 2003; 9(17):
6426–6431.22. Mhawech P, Herrmann F, Coassin M et al. Motility-related protein 1 (MRP-1/ CD9) expression in urothelial bladder carcinoma and its relation to tumor recurrence and progression. Cancer 2003; 98(8): 1649–1657. doi: 10.1002/ cncr.11698.
23. Hashida H, Takabayashi A, Tokuhara T et al. Clinical significance of transmembrane 4 superfamily in colon cancer. Br J Cancer 2003; 89(1): 158–167. doi: 10.1038/ sj.bjc.6601015.
24. Amatya VJ, Takeshima Y, Aoe K et al. CD9 expression as a favorable prognostic marker for patients with malignant mesothelioma. Oncol Rep 2013; 29(1): 21–28. doi: 10.3892/ or.2012.2116.
25. Kwon HJ, Choi JE, Kang SH et al. Prognostic significance of CD9 expression differs between tumour cells and stromal immune cells, and depends on the molecular subtype of the invasive breast carcinoma. Histopathology (2017); 70(7): 1155–1165. doi: 10.1111/ his.13184.
26. Kim T, Kim Y, Kwon HJ. Expression of CD9 and CD82 in papillary thyroid microcarcinoma and its prognostic significance. Endokrynol Pol (2019); 70(3): 224–231. doi: 10.5603/ EP.a2019.0009.
27. Lucarini G, Molinelli E, Licini C et al. Tetraspanin CD9 expression predicts sentinel node status in patients with cutaneous melanoma. Int J Mol Sci (2022); 23(9): 4775. doi: 10.3390/ ijms23094775.
28. Gregory CD, Rimmer MP. Extracellular vesicles arising from apoptosis: forms, functions, and applications. J Pathol 2023; 260(5): 592–608. doi: 10.1002/ path.6138.
29. Truong HH, Xiong J, Ghotra VPS et al. Beta1 integrin inhibition elicits a prometastatic switch through the TGFbeta-miR-200-ZEB network in E-cadherin-positive triple-negative breast cancer. Sci Signal 2014; 7(312): ra15. doi: 10.1126/ scisignal.2004751.
30. Yin H-L, Wu C-C, Lin C-H et al. Beta1 integrin as a prognostic and predictive marker in triple-negative breast cancer. Int J Mol Sci 2016; 17(9): 1432. doi: 10.3390/ ijms17091432.
31. Pleiko K, Haugas M, Parfejevs V et al. Targeting triple-negative breast cancer cells with a beta1-integrin binding aptamer. Mol Ther Nucleic Acids 2023; 33 : 871–884. doi: 10.1016/ j.omtn.2023.08.015.
32. Zhang W-J, Wang X-H, Gao S-T et al. Tumor-associated macrophages correlate with phenomenon of epithelial-mesenchymal transition and contribute to poor prognosis in triple-negative breast cancer patients. J Surg Res 2018; 222 : 93–101. doi: 10.1016/ j.jss.2017.09.035.
33. Tang D, Xu S, Zhang Q et al. The expression and clinical significance of the androgen receptor and E-cadherin in triple-negative breast cancer. Med Oncol 2012; 29(2): 526–533. doi: 10.1007/ s12032-011-9948-2.
34. Kashiwagi S, Yashiro M, Takashima T et al. Significance of E-cadherin expression in triple-negative breast cancer. Br J Cancer 2010; 103(2): 249–255. doi: 10.1038/ sj.bjc.6605735.
35. Kvokačková B, Fedr R, Kužílková D et al. Single-cell protein profiling defines cell populations associated with triple-negative breast cancer aggressiveness. Mol Oncol 2023; 17(6): 1024–1040. doi: 10.1002/ 1878-0261.13365.
Štítky
Detská onkológia Chirurgia všeobecná Onkológia
Článok vyšiel v časopiseKlinická onkologie
Najčítanejšie tento týždeň
2024 Číslo 1- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Fixní kombinace paracetamol/kodein nabízí synergické analgetické účinky
- Srovnání analgetické účinnosti metamizolu s ibuprofenem po extrakci třetí stoličky
- Nejasný stín na plicích – kazuistika
-
Všetky články tohto čísla
- Evropský plán boje proti rakovině a Mise rakovina – co nám přináší?
- Převodní systém srdeční jako nový rizikový orgán v radioterapii
- Střevní mikrobiota a karcinom pankreatu
- Molekulární podstata mnohočetného myelomu
- Evaluation pattern within tumor microenvironment and consequent gene expression in oral cancer
- Analysis of the effect of baseline detection and early clearance of ct-DNA, on survival outcomes among patients with advanced EGFR-mutant non-small cell lung cancer
- Immunohistochemical analysis of CD9, CD29 and epithelial to mesenchymal transition in triple-negative breast cancer
- Klinická zkušenost s kabozantinibem u pacientů s metastatickým karcinomem ledviny
- Léčba závislosti na tabáku u onkologických pacientů
- Pokročilé léčebné strategie metastatického kolorektálního karcinomu a karcinomu pankreatu
- MU Dr. Libor Havel (1967–2023)
- Doc. Ing. Čestmír Altaner, DrSc. oslávil vzácne životné jubileum – 90 rokov
- Poděkování recenzentům
- Klinická onkologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Molekulární podstata mnohočetného myelomu
- Střevní mikrobiota a karcinom pankreatu
- Analysis of the effect of baseline detection and early clearance of ct-DNA, on survival outcomes among patients with advanced EGFR-mutant non-small cell lung cancer
- Převodní systém srdeční jako nový rizikový orgán v radioterapii
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



