-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Family history of breast cancer and its association with disease severity and mortality
A family history (FH) of breast cancer (BC) is known to increase an individual's risk of disease onset. However, its role in disease severity and mortality is less clear. We aimed to ascertain associations between FH of BC, severity and BC-specific mortality in a hospital-based cohort of 5354 women with prospective information on FH. We included women diagnosed at Guy's and St Thomas’ NHS Foundation Trust between 1975 and 2012 (n = 5354). BC severity was defined and categorized as good, moderate, and poor prognosis. Data on BC-specific mortality was obtained from the National Cancer Registry and medical records. Associations between FH and disease severity or BC-specific mortality were evaluated using proportional odds models and Cox proportional hazard regression models, respectively. Available data allowed adjustment for potential confounders (e.g., treatment, socioeconomic status, and ethnicity). FH of any degree was not associated with disease severity at time of diagnosis (adjusted proportional OR: 1.00 [95% CI: 0.85 to 1.17]), which remained true also after stratification by period of diagnosis. FH of BC was not associated with BC-mortality HR: 0.99 (95% CI: 0.93 to 1.05). We did not find evidence to support an association between FH of BC and severity and BC-specific mortality. Our results indicate that clinical management should not differ between women with and without FH, when the underlying mutation is unknown.
Keywords:
breast cancer, Family history, mortality, severity
Authors: Jennifer C. Melvin 1; Wahyu Wulaningsih 1; Zac Hana 1; Arnie D. Purushotham 2,3; Sarah E. Pinder 2,3; Ian Fentiman 4; Cheryl Gillett 2; Anca Mera 1; Lars Holmberg 1,5,6; Mieke Van Hemelrijck 1,*
Authors place of work: Faculty of Life Sciences and Medicine, Division of Cancer Studies, Cancer Epidemiology Group, King's College London, London, United Kingdom 1; Faculty of Life Sciences and Medicine, Division of Cancer Studies, Section of Research Oncology, King's College London, London, United Kingdom 2; Guy's and St. Thomas’ NHS Foundation Trust, London, United Kingdom 3; Regional Cancer Centre, Uppsala/Orebro, Uppsala, Sweden 4; Research Oncology, Guy's Hospital, London, United Kingdom 5; Department of Surgical Sciences, Uppsala University, Uppsala, Sweden 6
Published in the journal: Cancer Medicine 2016; 5(5)
Category: Original Research
doi: https://doi.org/10.1002/cam4.648© 2016 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.Summary
A family history (FH) of breast cancer (BC) is known to increase an individual's risk of disease onset. However, its role in disease severity and mortality is less clear. We aimed to ascertain associations between FH of BC, severity and BC-specific mortality in a hospital-based cohort of 5354 women with prospective information on FH. We included women diagnosed at Guy's and St Thomas’ NHS Foundation Trust between 1975 and 2012 (n = 5354). BC severity was defined and categorized as good, moderate, and poor prognosis. Data on BC-specific mortality was obtained from the National Cancer Registry and medical records. Associations between FH and disease severity or BC-specific mortality were evaluated using proportional odds models and Cox proportional hazard regression models, respectively. Available data allowed adjustment for potential confounders (e.g., treatment, socioeconomic status, and ethnicity). FH of any degree was not associated with disease severity at time of diagnosis (adjusted proportional OR: 1.00 [95% CI: 0.85 to 1.17]), which remained true also after stratification by period of diagnosis. FH of BC was not associated with BC-mortality HR: 0.99 (95% CI: 0.93 to 1.05). We did not find evidence to support an association between FH of BC and severity and BC-specific mortality. Our results indicate that clinical management should not differ between women with and without FH, when the underlying mutation is unknown.
Keywords:
breast cancer, Family history, mortality, severityIntroduction
A family history of breast cancer (BC) is a known risk factor for the onset of disease [1], with an increased risk depending on the degree of family history [2, 3]. However, the link between family history and severity at time of diagnosis is less well described. Further clarification of this association could help the implementation of appropriate guidelines for clinicians and patients, with regards to screening, diagnosis and treatment. The association between family history and BC severity is unclear, and existing literature is scarce. A study of 2256 BC patients with invasive operable BC concluded that patients with a family history had smaller tumors that were more likely to be estrogen receptor (ER) positive [4]. However, a Swedish population-based registry study found a nonsignificant improved prognosis in women with a family history of BC [5]. Additionally, the association between family history and BC-specific mortality is not well defined. A review by the Collaborative Group on Hormonal Factors in Breast Cancer, constituting 52 studies, did not find an association between family history and BC-mortality [6] along with several others [7-12], whereas other studies have found a positive or even an inverse association [5, 13-20]. One study of over 1200 young women (defined as those diagnosed before the age of 45 years) with invasive BC found that women with no family history had a reduced BC-mortality. Interestingly, women with a first degree relation showed a 40% reduction in mortality risk, whereas women with only a second degree relation experienced similar results to women with no family history [17]. On the contrary, another study reported no statistically significant difference between patients with a family history of BC with regard to the risk of mortality[8]. Some of the variation may be explained by the varying study designs, study population sizes, and differences in the adjustment for confounding variables. Using data on 5354 BC patients from the King's Health Partners Breast Cancer Biobank (KHPBCBB), we assessed the extent to which family history of BC was associated with BC severity and BC-specific mortality.
Methods
Study population and data collection
The King's Health Partners Breast Cancer Biobank consists of over 12,000 patients, added consecutively with prospectively recorded data, who were treated for invasive BC at Guy's Hospital, London between January 1st 1975 and December 31st 2012. For the purposes of this study, we selected all women diagnosed with a primary invasive BC at Guy's Hospital, with data available on their family history. Patients were excluded if there was no documentation of curative surgery being undertaken, and if consent was not obtained. We also excluded 18 women who were lactating or pregnant at time of diagnosis, 1303 women with missing data for family history and 205 with an unknown menstrual status were not included. The final cohort contained 5354 women.
The following demographic and clinical characteristics, recorded prospectively, were included: age (≤40, 40–49, 50–59, 60–69 and ≥70), self-reported ethnicity (white, black, mixed, and other), socioeconomic status (SES) (scored as low, medium, high), parity (split into categories of 0, 1–2, 3–4, and ≥5 live births), natural menopausal status (pre, peri, and post), clinical tumor size (≤2 cm, 2–5 cm, and ≥5 cm), pathological lymph node status (none, <3, 4–10, and ≥10), histopathological grade, estrogen receptor, progesterone receptor, and HER2 status, and systemic treatments (including chemotherapy, endocrine therapy or radiotherapy). Socioeconomic status (SES) was based on the patients’ postcode using the ‘The English Indices of Deprivation 2010’, which is based on education, income, and wealth of environment. Family history was patient reported, and divided into first degree, second degree, first and second degree and finally, third degree and no history of BC. Patients with only a third degree of family history were grouped with those without any family history because evidence is limited, but the existing consensus is that the associated increased risk of BC is smaller in patients with only a third degree relative with a history of BC [1]. BC severity was defined using a modified version of the St Gallen criteria, as shown in Table 1 [21].
Tab. 1. Breast cancer (BC) severity criteria, created using a modified version of the St Gallen criteria [21]. ![Breast cancer (BC) severity criteria, created using a modified version of the St Gallen criteria [21].](https://www.prelekara.sk/media/cache/resolve/media_object_image_small/media/image/32a93071ae1fc8d77b8f187fdea10ad9.png)
Clinical follow-up information was collected for all women as the hospital's standard procedure up until the 31st December 2007. After 2007, low-risk women were not followed up as per hospital protocol, but only those who later returned to clinic with evidence of further disease were followed up. Women who did not return to the clinic were assumed to have not experienced any disease progression and/or recurrence. All follow-up information was taken from medical records, which also provided information on cause of death, date of last contact, and date of disease recurrence. National death certificate information was obtained from the National Cancer Registry, and the Office of National Statistics, which were used to confirm the cause of death. Data collection for the purposes of this study was permitted under the approval of the Guy's NHS Research Ethics Committee (REC Number: 12/EE/0493) for women diagnosed up to September 2006, for women diagnosed afterwards individual consent was obtained.
Statistical analysis
The association between family history and BC severity at the time of diagnosis was analyzed with multivariate proportional odds models, as the latter is an ordinal categorical outcome measurement. All models were checked to ensure that the proportional odds assumption held. As a next step, multivariate Cox proportional hazards models were used to assess the association between family history and BC-specific death. Follow-up time was defined from the time of diagnosis until death or last day seen. All models were adjusted for age at diagnosis, time period of diagnosis, ethnicity, SES, parity, menopausal status, positive nodes, tumor size, ER, PR, HER2, as well as primary treatment unless stated otherwise. Stratified analyses were performed by time period of diagnosis (1975–1989 or 1989–2012). This stratification aimed to capture the effects of the introduction of mammography screening [22]. All statistical analyses were performed with Statistical Analysis Systems (SAS) release 9.3 (SAS Institute, Cary, NC).
Results
Of the 5354 included women, 3428 (64%) had information sufficient to categorize them by severity at time of diagnosis. Of these women categorized by severity, 791 (23%) had a family history of BC: patients with only a first degree accounted for 428 (54%), while those with second degree numbered 286 (36%) and finally, first and second degree accounted for 77 (10%). Thus, leaving 1926 women (36%) with either no family history of BC as defined by first or second. Patient characteristics of women with information for severity, and women without are shown in Table 2: there were no significant differences between them. Furthermore, there was no significant difference in the mean age at primary diagnosis between women with a family history (56.04 [SD ± 12]) and women without (654.52 [SD ± 11.9]).
Tab. 2. Descriptive characteristics by family history. 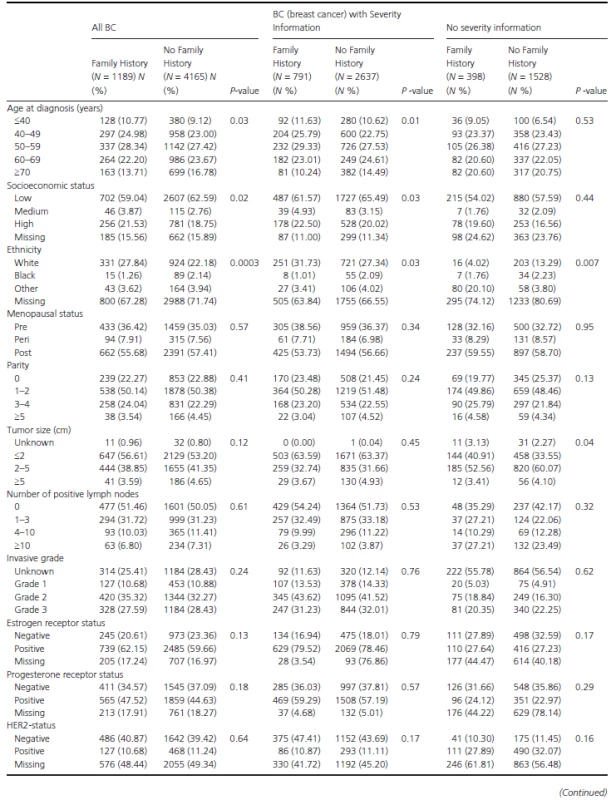
Table 3 shows the study population's demographics stratified by BC severity status. No significant differences were observed between women with and without a family history. As a next step, the association between family history and BC severity (at time of diagnosis) was assessed through proportional odds ratios (OR) (Table 4). No ORs observed were statistically significant. For example, when comparing women with any family history to women without family history the proportional OR was 1.00 (95% CI: 0.85–1.17). To investigate this association further, analyses were stratified by time period of diagnosis, but this did not alter any of the findings (Table 4).
Tab. 3. Descriptive table of breast cancer prognosis (at the time of diagnosis, as defined in the methods) by family history of breast cancer as well as diagnosis period. 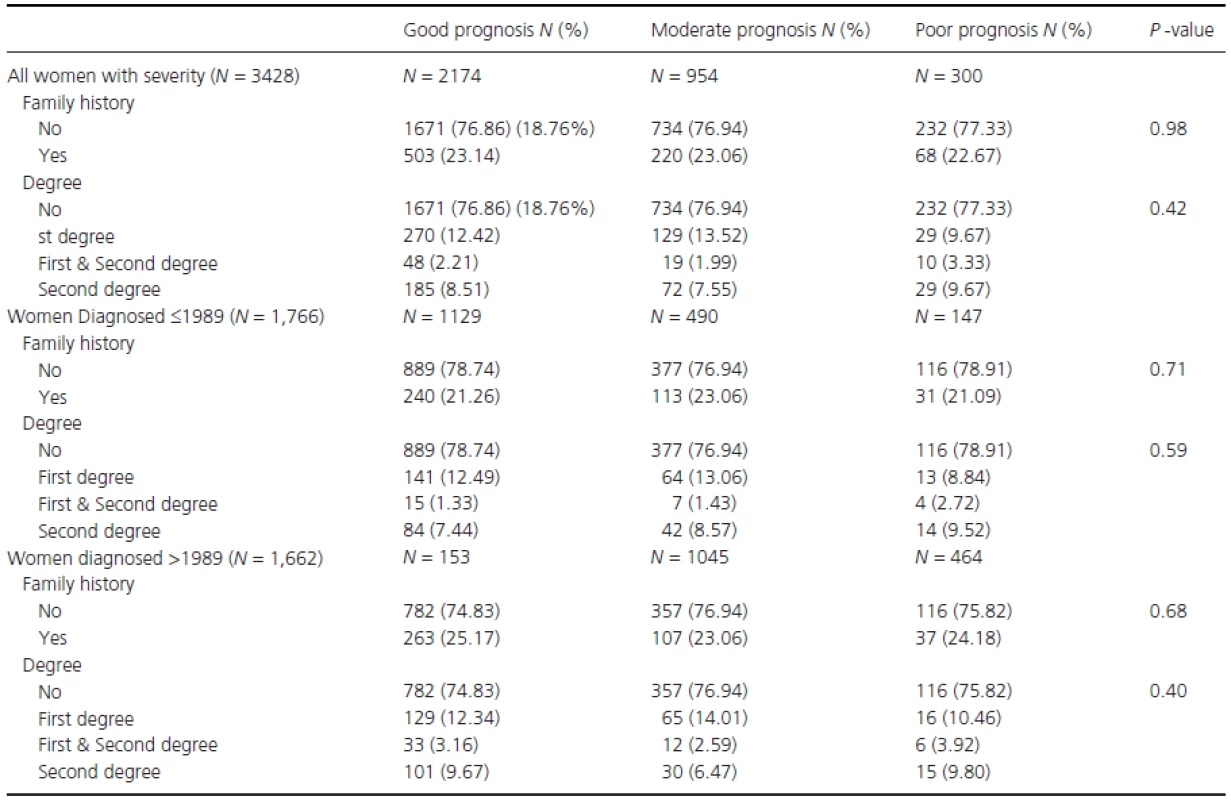
Tab. 4. Proportional odds ratios (OR) and 95% confidence intervals (CI) by breast cancer severity at diagnosis, for women in the GSTT database, adjusted for time period of diagnosis, ethnicity, and socioeconomic status. 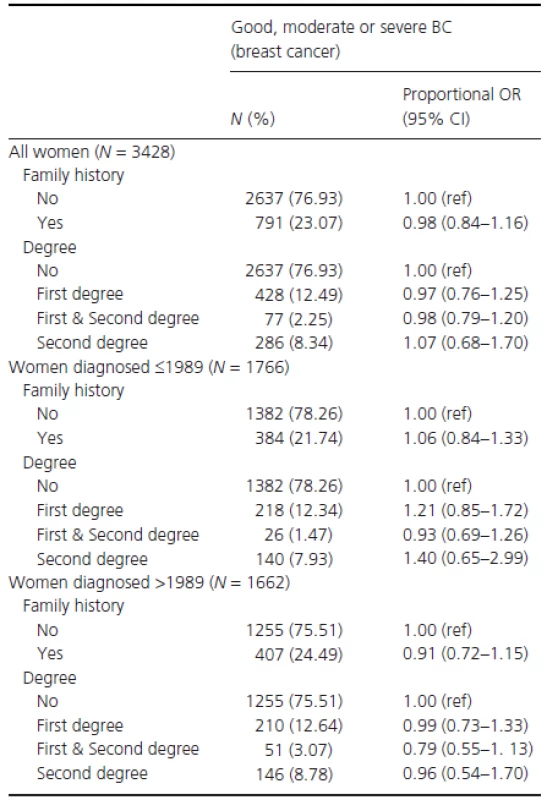
Finally, the hazard ratio (HR) for the risk of fatal BC was investigated (Table 5). No association between FH - and BC-specific death was observed (e.g., crude model HR: 0.95 [95% CI: 0.90–1.01]). Similarly, the same was observed when varying degrees of family history were investigated individually (e.g., HR for women with first degree of family history: 0.99 (95% CI: 0.93–1.05) adjusted for age, ethnicity, SES, parity, menopausal status, invasive grade, positive nodes, tumor size, PR, ER, HER2 receptors, surgery type, and adjuvant treatments).
Tab. 5. Hazard ratio (HR) and 95% confidence intervals (CI) for fatal BC (breast cancer) by family history of breast cancer, in women with sufficient data to allow classification by severity. 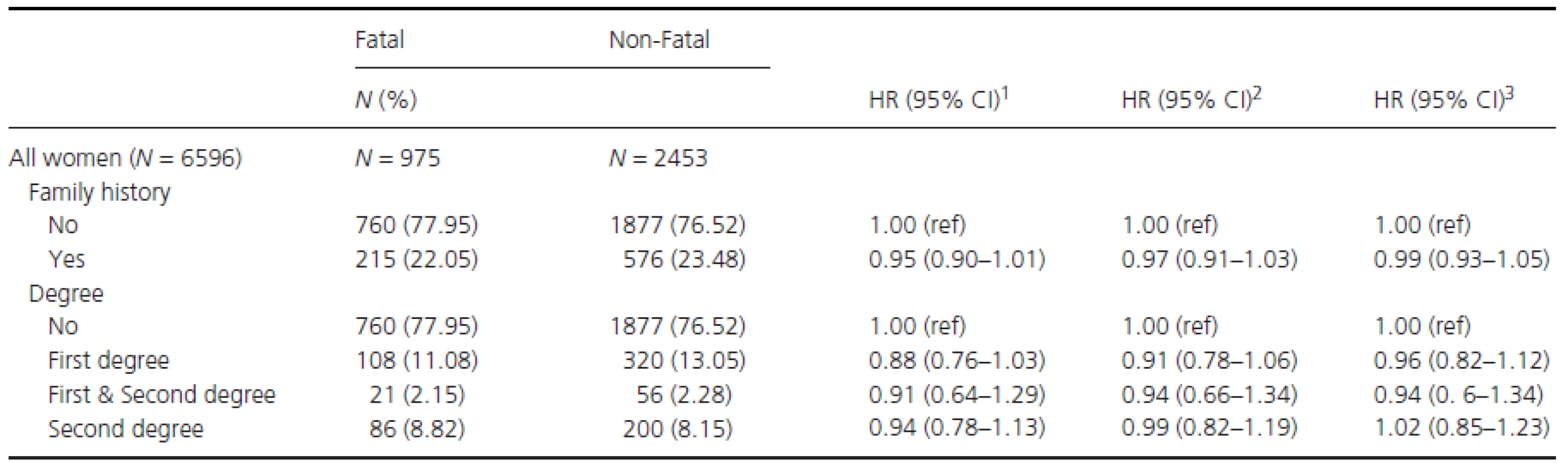
1Crude model. 2Adjusted for age, time period of diagnosis, ethnicity, and socioeconomic status (SES). 3Adjusted for age, time period of diagnosis, ethnicity, SES, parity, menopausal status, invasive grade, positive nodes, tumor size, PR, ER (estrogen receptor), HER2 receptors, surgery type and adjuvant treatments. Discussion
Given contradicting findings within the existing literature regarding associations between family history and both tumor characteristics and BC-specific death, we used our hospital-based cohort to investigate this further. The results from this study demonstrated that family history does not appear to have an association with BC severity at time of diagnosis, nor BC-mortality.
Literature indicates women with an early-onset BC with family history have a higher frequency of BRCA1 and BRCA2 compared to women without a family history [23, 24]. Accumulating evidence has shown that different tumor characteristics were identified in patients with theBRCA1 mutation – less so in patients with the BRCA2 mutation - in comparison to patients without the mutation [25-30]. Survival advantage for BC cases with a BRCA1 mutation has been reported [31, 32], whereas another study found poor survival when the patient's had BRCA1mutation [33].
There is discrepancy in the associations between family history and BC prognosis reported to date. A study incorporating Utah's Population Database found that women under the age of 50 with a family history of BC were statistically significantly at a greater risk of a poorer survival when compared to those women without; with a relative risk of 1.54 (95% CI: 0.98–2.41) for women with first degree relatives previously diagnosed with BC [34]. In contrast, a recent population-based study [35] based in Netherlands reported a lower risk of all-cause death with ≥2 first-degree relative with family history (HR 0.2; 95% CI 0.06–1.0). Nevertheless, several studies have found no association between family history and BC overall survival [8, 36, 37]. For instance, a study based on a Canadian population-based familial breast cancer registry [38] reported that a family history of breast or ovarian cancer was not associated with recurrence or death. Chang and colleagues also reported a null association with all-cause death in a cohort of BC patients in the Breast Cancer Family Registry in Northern California, USA when excluding those with BRCA1 and BRCA2 mutation [8]. Therefore, findings from our study are also in agreement with the majority of existing research regarding BC-mortality. Of the studies demonstrating a statistical difference in mortality, most of them focused on younger women, and tended to have smaller study populations.
With regard to disease-free survival, recent findings from the UK Prospective Outcomes in Sporadic versus Hereditary breast cancer (POSH) suggested that there is no difference in disease progression between female BC patients with or without a family history, with a HR of 0.89 (95% CI: 0.76–1.03) [39]. It is clinically relevant to know that women with a family history of BC are not necessarily at a greater risk. Thus, no evidence is provided to date to suggest that these women should be given treatment different to that of what current protocol dictates.
The strength of this hospital-based cohort is the ability to adjust for possible confounding. For example, adjustments could be made to account for more aggressive treatments, or any confounding by socioeconomic status or ethnicity. Furthermore, while the cohort is hospital based, it is defined by a specific catchment area so that the included individuals remain unselected and it is close to a population-based cohort. A study based largely on referred individuals, may have led to a bias of different family history. In previous studies where positive associations were observed between FH and severity or mortality limited adjustments for confounding were made [1, 4, 5, 14, 18]. By contrast, studies which observed no association between the two, as seen here, adjusted for established prognostic factors along with age, and race in some instances [8-10, 37].
Despite its large size, there are some limitations to consider. Firstly, patient genetics were not collected in our study. Thus, we cannot disentangle any effect modifications by type of mutation (i.e., BRCA1). Secondly, approximately one-third of women in the dataset did not have information on BC severity recorded. However, it can be seen in Table 2 that the women with severity are representative of the entire cohort. A further limitation of this dataset is the incomplete data collection for some confounding variables, such as ER/PR/HER2 status. However, due to the large population and extensive follow-up period, it remains a valuable and informative resource. Another potential limitation of this dataset is the lack of additional information for the known risk factors of BC, such as age at menarche, age at first birth, obesity, and HRT. However, these risk factors (bar obesity) are not among those considered to be strong prognostic factors of BC and thus we deem the potential confounding influence to be low [40-42]. In terms of disease severity, a modified version of the St Gallen's criteria was used where we incorporated information on age (above or below 40 years at the time of diagnosis), ER status as well as histopathological grade. A strength here is that these elements were prospectively derived from our KHPBCBB database. Furthermore, while only invasive tumors were included, we did not stratify by individual tumor type due to power issues resulting from smaller numbers. Finally, the long-time span of recruitments could be viewed as a potential variable to introduce bias, however, we believe that by adjusting for potential confounders such as treatment, surgery, age and diagnosis, and the diagnosis period, that the potential for any influence on the findings is small.
Conclusion
We did not find evidence to support an association between family history of BC and severity and BC-specific mortality. Our results indicate that clinical management should not differ between women with and without family history when the underlying mutation(s) is (are) unknown. The literature indicates that it may be different for BRCA1/2 carriers.
Conflict of Interest
None declared.
Funding Information
This research was supported by the Experimental Cancer Medicine Centre at King’s College London and also by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St. Thomas’s NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Received: 22 July 2015
Revised: 22 December 2015
Accepted: 29 December 2015
Version of Record online: 22 January 2016* Correspondence:
Mieke Van Hemelrijck
Faculty of Life Sciences and Medicine, Division of Cancer Studies, King's College London, Cancer Epidemiology Group, Research Oncology, 3rd Floor, Bermondsey Wing, Guy's Hospital
London, SE1 9RT, United KingdomTel: +44 (0)20 7188 7904
Fax: 02071889986
E-mail: mieke.vanhemelrijck@kcl.ac.uk
Zdroje
1 Slattery, M. L., and R. A. Kerber. 1993. A comprehensive evaluation of family history and breast cancer risk. The Utah Population Database. Jama. 270 : 1563–8.
2 Pharoah, P. D., N. E. Day, S. Duffy, D. F. Easton, and B. A. Ponder. 1997. Family history and the risk of breast cancer: a systematic review and meta-analysis. Int. J. Cancer 71 : 800–9.
3 Ramsey, S. D., P. Yoon, R. Moonesinghe, and M. J. Khoury. 2006. Population-based study of the prevalence of family history of cancer: implications for cancer screening and prevention. Genet. Med. 8 : 571–5.
4 Molino, A., M. Giovannini, R. Pedersini, M. Frisinghelli, R. Micciolo, M. Mandara, et al. 2004. Correlations between family history and cancer characteristics in 2256 breast cancer patients. Br. J. Cancer 91 : 96–8.
5 Thalib, L., S. Wedren, F. Granath, H. O. Adami, B. Rydh, C. Magnusson, et al. 2004. Breast cancer prognosis in relation to family history of breast and ovarian cancer. Br. J. Cancer 90 : 1378–81.
6 Collaborative Group on Hormonal Factors in Breast. 2001. C. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet 358 : 1389–99.
7 Anderson, D. E., and M. D. Badzioch. 1986. Survival in familial breast cancer patients. Cancer 58 : 360–5.
8 Chang, E. T., R. L. Milne, K. A. Phillips, J. C. Figueiredo, M. Sangaramoorthy, T. H. Keegan, et al. 2009. Family history of breast cancer and all-cause mortality after breast cancer diagnosis in the Breast Cancer Family Registry. Breast Cancer Res. Treat. 117 : 167–76.
9 Israeli, D., P. I. Tartter, S. T. Brower, B. Mizrachy, and J. Bratton. 1994. The significance of family history for patients with carcinoma of the breast. J. Am. Coll. Surg. 179 : 29–32.
10 Ruder, A. M., P. F. Moodie, N. A. Nelson, and N. W. Choi. 1988. Does family history of breast cancer improve survival among patients with breast cancer? Am. J. Obstet. Gynecol. 158 : 963–8.
11 Russo, A., A. Herd-Smith, D. Gestri, S. Bianchi, V. Vezzosi, M. Rosselli Del Turco, et al. 2002. Does family history influence survival in breast cancer cases? Int. J. Cancer 99 : 427–30.
12 Schouten, L. J., P. S. Hupperets, J. J. Jager, L. Volovics, J. A. Wils, A. L. Verbeek, et al. 1997. Prognostic significance of etiological risk factors in early breast cancer. Breast Cancer Res. Treat. 43 : 217–23.
13 Albano, W. A., J. A. Recabaren, H. T. Lynch, A. S. Campbell, J. A. Mailliard, C. H. Organ, et al. 1982. Natural history of hereditary cancer of the breast and colon. Cancer 50 : 360–3.
14 Fukutomi, T., Y. Kobayashi, T. Nanasawa, H. Yamamoto, and H. Tsuda. 1993. A clinicopathological analysis of breast cancer in patients with a family history. Surg. Today 23 : 849–54.
15 Langlands, A. O., G. R. Kerr, and S. M. Bloomer. 1976. Familial breast cancer. Clin. Oncol. (R. Coll. Radiol.) 2 : 41–5.
16 Lynch, H. T., W. A. Albano, J. A. Recabaren, P. R. Fain, and P. M. Lynch.1981. Survival in hereditary breast and colon cancer. JAMA: J. American Med. Assoc.. 246 : 1197.
17 Malone, K. E., J. R. Daling, D. R. Doody, C. O'Brien, A. Resler, E. A. Ostrander, et al. 2011. Family history of breast cancer in relation to tumor characteristics and mortality in a population-based study of young women with invasive breast cancer. Cancer Epidemiol. Biomarkers Prev. 20 : 2560–71.
18 Malone, K. E., J. R. Daling, N. S. Weiss, B. McKnight, E. White, and L. F. Voigt. 1996. Family history and survival of young women with invasive breast carcinoma. Cancer 1 : 1417–25.
19 Mohammed, S. N., P. Smith, S. V. Hodgson, I. S. Fentiman, D. W. Miles, D. M. Barnes, et al. 1998. Family history and survival in premenopausal breast cancer. Br. J. Cancer 77 : 2252–6.
20 Wobbes, T., M. P. van de Wiel, R. F. van der Sluis, and A. G. Theeuwes. 1987. The effect of familiality on clinical presentation and survival in mammary carcinoma. Eur. J. Surg. Oncol. 13 : 119–21.
21 Goldhirsch, A., J. H. Glick, R. D. Gelber, A. S Coates, and H. J. Senn. 2001. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. Seventh International Conference on Adjuvant Therapy of Primary Breast Cancer. J. Clin. Oncol. 19 : 3817–27.
22 van Schoor, G., S. M. Moss, J. D. Otten, R. Donders, E. Paap, G. J. den Heeten, et al. 2011. Increasingly strong reduction in breast cancer mortality due to screening. Br. J. Cancer 104 : 910–4.
23 Loman, N., O. Johannsson, U. Kristoffersson, H. Olsson, and A. Borg. 2001. Family history of breast and ovarian cancers and BRCA1 and BRCA2 mutations in a population-based series of early-onset breast cancer. J. Natl Cancer Inst. 93 : 1215–23.
24 Malone, K. E., J. R. Daling, C. Neal, N. M. Suter, C. O'Brien, K. Cushing-Haugen, et al. 2000. Frequency of BRCA1/BRCA2 mutations in a population-based sample of young breast carcinoma cases. Cancer 88 : 1393–402.
25 Pathology of familial breast cancer : differences between breast cancers in carriers of BRCA1 or BRCA2 mutations and sporadic cases.. 1997. Breast Cancer Linkage Consortium. Lancet 349 : 1505–10.
26 Adem, C., C. Reynolds, C. L. Soderberg, J. M. Slezak, S. K. McDonnell, T. J. Sebo, et al. 2003. Pathologic characteristics of breast parenchyma in patients with hereditary breast carcinoma, including BRCA1 and BRCA2 mutation carriers. Cancer 97 : 1–11.
27 Chappuis, P. O., V. Nethercot, and W. D. Foulkes. 2000. Clinico-pathological characteristics of BRCA1 - and BRCA2-related breast cancer. Semin. Surg. Oncol. 18 : 287–95.
28 Honrado, E., J. Benitez, and J. Palacios. 2006. Histopathology of BRCA1 - and BRCA2-associated breast cancer. Crit. Rev. Oncol. Hematol. 59 : 27–39.
29 Lakhani, S. R., J. Jacquemier, J. P. Sloane, B. A. Gusterson, T. J. Anderson, M. J. van de Vijver, et al. 1998. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J. Natl Cancer Inst. 90 : 1138–45.
30 Palacios, J., E. Honrado, A. Osorio, A. Cazorla, D. Sarrio, A. Barroso, et al. 2003. Immunohistochemical characteristics defined by tissue microarray of hereditary breast cancer not attributable to BRCA1 or BRCA2 mutations: differences from breast carcinomas arising in BRCA1 and BRCA2 mutation carriers. Clin. Cancer Res. 9(10 Pt 1):3606–14.
31 Porter, D. E., B. B. Cohen, M. R. Wallace, E. Smyth, U. Chetty, J. M. Dixon, et al. 1994. Breast cancer incidence, penetrance and survival in probable carriers of BRCA1 gene mutation in families linked to BRCA1 on chromosome 17q12-21. Br. J. Surg. 81 : 1512–5.
32 Rubin, S. C., I. Benjamin, K. Behbakht, H. Takahashi, M. A. Morgan, V. A. LiVolsi, et al. 1996. Clinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA1. N. Engl. J. Med. 7 : 1413–6.
33 Foulkes, W. D., N. Wong, J. S. Brunet, L. R. Begin, J. C. Zhang, J. J. Martinez, et al. 1997. Germ-line BRCA1 mutation is an adverse prognostic factor in Ashkenazi Jewish women with breast cancer. Clin. Cancer Res. 3(12 Pt 1):2465–9.
34 Slattery, M. L., T. D. Berry, and R. A. Kerber. 1993. Is survival among women diagnosed with breast cancer influenced by family history of breast cancer? Epidemiology 4 : 543–8.
35 Jobsen, J. J., J. van der Palen, M. Brinkhuis, F. Ong, and H. Struikmans. 2015. Long-term effects of first degree family history of breast cancer in young women: Recurrences and bilateral breast cancer. Acta Oncol. 23 : 1–6.
36 Breast Cancer Care. 2010. http://www.nhs.uk/ipgmedia/national/Breast%20Cancer%20Care/Assets/InvasivelobularbreastcancerBCC11pages.pdf Invasive Lobular Breast Cancer: Factsheet. London
37 Margolin, S., H. Johansson, L. E. Rutqvist, A. Lindblom, and T. Fornander. 2006. Family history, and impact on clinical presentation and prognosis, in a population-based breast cancer cohort from the Stockholm County. Fam. Cancer 5 : 309–21.
38 Figueiredo, J. C., M. Ennis, J. A. Knight, J. R. McLaughlin, N. Hood, F. O'Malley, et al. 2007 Sep. Influence of young age at diagnosis and family history of breast or ovarian cancer on breast cancer outcomes in a population-based cohort study. Breast Cancer Res. Treat. 105 : 69–80.
39 Eccles, B. K., E. R. Copson, R. I. Cutress, T. Maishman, D. G. Altman, P. Simmonds, et al. 2015 Jul. Family history and outcome of young patients with breast cancer in the UK (POSH study). Br. J. Surg. 102 : 924–35.
40 Arpino, G., M. Milano, and S. De Placido. 2015. Features of aggressive breast cancer. Breast 24 : 594–600.
41 Hao, S., Y. Liu, K. D. Yu, S. Chen, W. T. Yang, and Z. M. Shao. 2015. Overweight as a Prognostic Factor for Triple-Negative Breast Cancers in Chinese Women. PLoS ONE 10:e0129741.
42 Park, S., W. Han, J. Kim, M. K. Kim, E. Lee, T. K. Yoo, et al. 2015. Risk Factors Associated with Distant Metastasis and Survival Outcomes in Breast Cancer Patients with Locoregional Recurrence. J. Breast Cancer 18 : 160–6.
Štítky
Onkológia
Článok vyšiel v časopiseCancer Medicine
Najčítanejšie tento týždeň
2016 Číslo 5- Nejasný stín na plicích – kazuistika
- Zpracované masné výrobky a červené maso jako riziko rozvoje kolorektálního karcinomu u žen? Důkazy z prospektivní analýzy
- DESATORO PRE PRAX: Aktuálne odporúčanie ESPEN pre nutričný manažment u pacientov s COVID-19
- I „pouhé“ doporučení znamená velkou pomoc. Nasměrujte své pacienty pod křídla Dobrých andělů
- Když se ve střevech děje něco nepatřičného...
Najčítanejšie v tomto čísle- Acute pancreatitis as a complication of childhood cancer treatment
- Family history of breast cancer and its association with disease severity and mortality
- The effects of hemoglobin levels and their interactions with cigarette smoking on survival in nasopharyngeal carcinoma patients
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy




