-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Phylogenomics of Ligand-Gated Ion Channels Predicts Monepantel Effect
The recently launched veterinary anthelmintic drench for sheep (Novartis Animal Health Inc., Switzerland) containing the nematocide monepantel represents a new class of anthelmintics: the amino-acetonitrile derivatives (AADs), much needed in view of widespread resistance to the classical drugs. Recently, it was shown that the ACR-23 protein in Caenorhabditis elegans and a homologous protein, MPTL-1 in Haemonchus contortus, are potential targets for AAD action. Both proteins belong to the DEG-3 subfamily of acetylcholine receptors, which are thought to be nematode-specific, and different from those targeted by the imidazothiazoles (e.g. levamisole). Here we provide further evidence that Cel-ACR-23 and Hco-MPTL-1-like subunits are involved in the monepantel-sensitive phenotype. We performed comparative genomics of ligand-gated ion channel genes from several nematodes and subsequently assessed their sensitivity to anthelmintics. The nematode species in the Caenorhabditis genus, equipped with ACR-23/MPTL-1-like receptor subunits, are sensitive to monepantel (EC50<1.25 µM), whereas the related nematodes Pristionchus pacificus and Strongyloides ratti, which lack an ACR-23/MPTL-1 homolog, are insensitive (EC50>43 µM). Genome sequence information has long been used to identify putative targets for therapeutic intervention. We show how comparative genomics can be applied to predict drug sensitivity when molecular targets of a compound are known or suspected.
Published in the journal: Phylogenomics of Ligand-Gated Ion Channels Predicts Monepantel Effect. PLoS Pathog 6(9): e32767. doi:10.1371/journal.ppat.1001091
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001091Summary
The recently launched veterinary anthelmintic drench for sheep (Novartis Animal Health Inc., Switzerland) containing the nematocide monepantel represents a new class of anthelmintics: the amino-acetonitrile derivatives (AADs), much needed in view of widespread resistance to the classical drugs. Recently, it was shown that the ACR-23 protein in Caenorhabditis elegans and a homologous protein, MPTL-1 in Haemonchus contortus, are potential targets for AAD action. Both proteins belong to the DEG-3 subfamily of acetylcholine receptors, which are thought to be nematode-specific, and different from those targeted by the imidazothiazoles (e.g. levamisole). Here we provide further evidence that Cel-ACR-23 and Hco-MPTL-1-like subunits are involved in the monepantel-sensitive phenotype. We performed comparative genomics of ligand-gated ion channel genes from several nematodes and subsequently assessed their sensitivity to anthelmintics. The nematode species in the Caenorhabditis genus, equipped with ACR-23/MPTL-1-like receptor subunits, are sensitive to monepantel (EC50<1.25 µM), whereas the related nematodes Pristionchus pacificus and Strongyloides ratti, which lack an ACR-23/MPTL-1 homolog, are insensitive (EC50>43 µM). Genome sequence information has long been used to identify putative targets for therapeutic intervention. We show how comparative genomics can be applied to predict drug sensitivity when molecular targets of a compound are known or suspected.
Introduction
Nematode parasites of sheep represent one of the major constraints in the wool, meat and milk industries world wide [1]. The gastro-intestinal parasite Haemonchus contortus, in particular, causes substantial losses. In the mid-1950s, the existence of anthelmintic resistant worm populations came to light with the failure of phenothiazine against Haemonchus [2]. Since then, nematode populations resistant to the three classical groups of anthelmintics, i.e. the benzimidazoles, imidazothiazoles and macrocyclic lactones, and combinations of these have been described [3]–[7]. Recently, the amino-acetonitrile derivatives (AADs) have been reported as a new class of synthetic anthelmintics active against gastro-intestinal nematodes of sheep [8] and a first drug from this family, monepantel, was, at the time of writing licensed to market in Australasia, Europe and Latin America (ZOLVIX; Novartis Animal Health Inc., Switzerland; [9]). Investigations to understand the mode of action of the AADs in Caenorhabditis elegans have been performed using chemical mutagenesis and gene mapping via a genetic recombination approach. Out of 44 isolated resistant alleles, 36 were mapped to a single gene, acr-23, designating it as a major contributor to the AAD response in C. elegans [8]. A further study on mutant H. contortus isolates identified the gene monepantel-1 (Hco-mptl-1) as a major target candidate for AADs action in this species [8], [10]. Both Cel-acr-23 and Hco-mptl-1 are predicted to encode a nicotinic acetylcholine receptor (nAChR) subunit. These belong to the superfamily of ligand-gated ion channel (LGIC) subunits. These are the modular components with the ability to form a large number of channels with different properties through heteromultimerisation (see e.g. [11]), as all characterised LGIC function as penta - or tetramers. They provide many important drug and toxin targets: levamisole and pyrantel act as agonists of nAChR [12], paraherquamide as a competitive antagonist of these channels [13] and ivermectin modulates glutamate-gated chloride channels [12], [14], [15]. ACR-23 and MPTL-1 are members of the DEG-3 subfamily of acetylcholine receptor subunit genes and distinct from those targeted by imidazothiazoles [16], [17]. Members of this subfamily have so far only been found in nematodes and no cross-resistance between the AADs and the imidazothiazoles have been documented [8].
All animals appear to have about the same number of nAChR α subunits (around 16), with exception of the nematodes [18]. Among the completely sequenced genomes from animals, those with the highest (C. elegans) and smallest (Brugia malayi) numbers of such genes are both from the nematodes [19]. The reason for the variation in numbers of nAChR α subunits and other LGIC subunits is not clear. However, certain LGIC subunits form heteromultimeric channels that provide prominent anthelmintic-specific drug targets (highlighted in Figure 1), in particular for ivermectin (AVR-14, AVR-15, GLC-1), levamisole (UNC-38, UNC-29, UNC-63, LEV-1, LEV-8) and monepantel (MPTL-1). Several new, draft nematode genomes as well as pre-publication quality assemblies are now available from ongoing or recently finished sequencing projects (see Table 1).
Fig. 1. Phylogenetic tree based on the LBD region of putative LGIC genes. 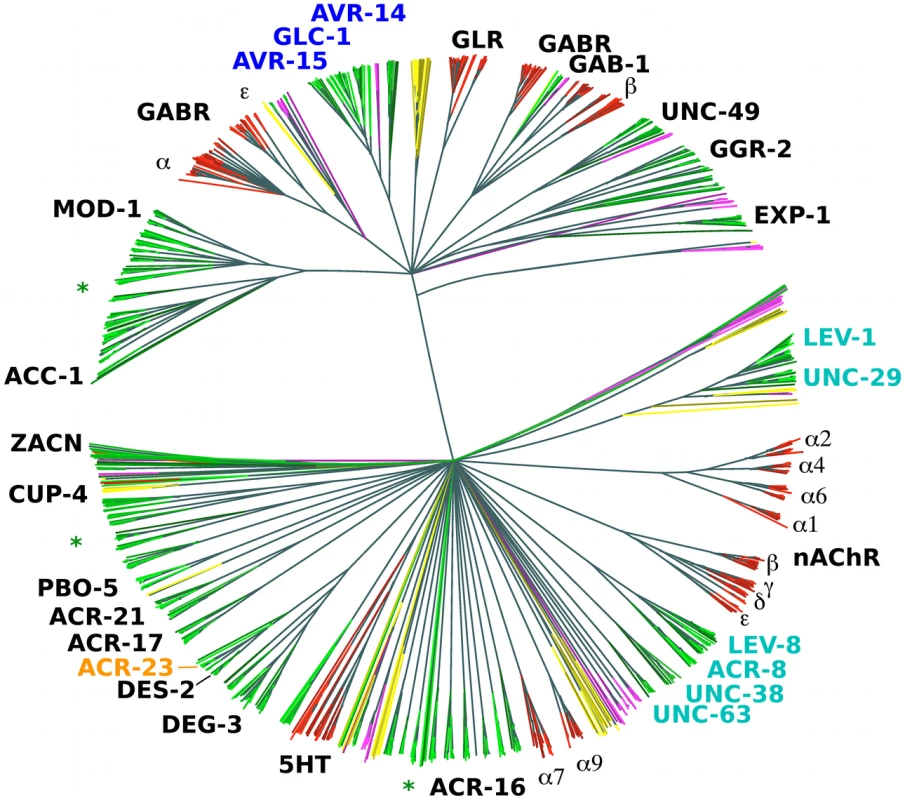
NJ tree (clustalw) from the LBD region of conceptually translated putative LGIC genes as detected with Genewise after an initial Blast screen with the 210 seeds (see Materials and Methods) - 1426 sequences in total. Thousand bootstrap iterations were performed and branches below 50% bootstrap support were collapsed. Nematode sequences are shown in shades of green, platyhelminthes yellow, insect purple and vertebrate red. Some C. elegans and human subunits are labelled, and the labels for proteins involved in drug susceptibility are coloured: levamisole - cyan, monepantel - orange and ivermectin – blue. Green asterisks indicate branches that similar to these latter appear broad and nematode specific and could be attractive for further investigation as targets for other compounds. Tab. 1. Genome sequences included in tree construction from indicated phylum. 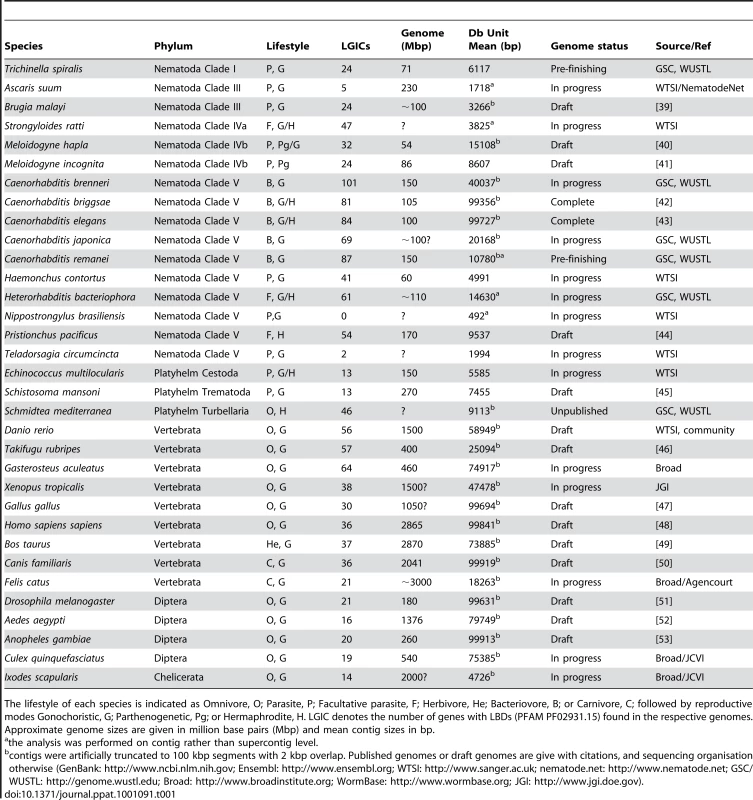
The lifestyle of each species is indicated as Omnivore, O; Parasite, P; Facultative parasite, F; Herbivore, He; Bacteriovore, B; or Carnivore, C; followed by reproductive modes Gonochoristic, G; Parthenogenetic, Pg; or Hermaphrodite, H. LGIC denotes the number of genes with LBDs (PFAM PF02931.15) found in the respective genomes. Approximate genome sizes are given in million base pairs (Mbp) and mean contig sizes in bp. To learn what parts of the LGIC superfamily are unique to nematodes and in consideration of much new sequence information, we constructed a simple phylogenomic pipeline to further understand the mechanisms behind the action of monepantel. We explored the LGIC superfamily by in silico searches, and while we found a considerable number of tentative new family members since the last such survey was made [19], the DEG-3-subfamily remains nematode specific. In an in vitro drug assay we further show that susceptibility to the AADs directly follows the presence of ACR-23 or MPTL-1 homologs in the genomes from the nematodes investigated.
Materials and Methods
Genome analysis
Genome data in the form of contig or supercontig DNA sequence fasta files were downloaded from GenBank (NCBI), Ensembl, WTSI, nematode.net, GSC/WUSTL, Broad and WormBase (all attributed and referenced in Table 1). Sequences from genomes with long contiguous sequences were artificially divided into 100 kb segments (indicated by asterisks in Table 1). Seed sequences were obtained as peptide fasta files from WormBase [20] and Uniprot [21].
A Blast [22] screen with the seed sequences as queries against the genomic sequence databases was performed. Only contigs with hits (E<0.1) were searched by Genewise [23] with the PFAM [24] motifs LGIC_LBD (PF02931.15) and LGIC_MEMB (PF02932.8) for global scoring (ls). Splice sites were considered using the Genewise-provided worm gene model. The seed peptide sequences were searched using the same PFAM profiles but with hmmsearch from the hmmer2 package (by Eddy, http://hmmer.janelia.org). The protein domains, conceptually translated from DNA or directly from the seed proteins, that exhibited E-values below the trusted E-value cut-off were aligned and an nj tree (bootstrap 1000 iterations) was constructed with clustalw [25]. These steps were automated in bash and Perl using tools from the EMBOSS package [26] and executed on LINUX computers using less than 1.5Gb RAM. Trees were visualised with Dendroscope [27] and HyperTree [28]. For Figure 1, furcations with bootstrap support below 50% were fused in jtreeview (Frickey, Lupas http://www.eb.tuebingen.mpg.de/departments/1-protein-evolution/software/jtreeview/). Co-segregation with known named seed sequences in the bootstrapped tree was used for assigning putative identity to homologous genes. Trees based on available, confirmed or predicted, full-length protein sequences (WormBase WS195 C. brenneri, C. elegans, C. briggsae, C. japonica, C. remanei, Pristionchus pacificus and B. malayi) were also constructed. The same seed sequences were used to pick predicted genes with a blastp similarity (E<0.01) for inclusion in a profile search and tree construction using the aforementioned methods.
Nematode strains
Nematode strains C. briggsae AF16, C. brenneri PB2801, C. remanei PB4641, C. japonica DF5081 and P. pacificus PS312 as well as the mutants VC1598: Cel-acr-20(ok1849)/mT1 II; +/mT1[dpy-10(e128)] III, NC293: Cel-acr-5(ok180) III, TU1803: Cel-deg-3(u662) Cel-des-2(u695) V and RB1226: Cel-acr-18(ok1285) V were obtained from the Caenorhabditis Genetics Center (CGC), Minneapolis, USA, which is funded by the NIH National Center for Research Resources (NCRR). Caenorhabditis elegans Bristol N2 and AP134: Cel-acr-23 (cb27) V [8] were kind gifts from Prof. Alessandro Puoti, University of Fribourg. Nematodes were maintained at 20°C on Nematode Growth Medium (NGM) plates (3 g NaCl, 17 g Agar, 2.5 g peptone in 975 ml H2O, autoclaved, added 1 ml cholesterol (Sigma) prepared to 5 mg/ml in EtOH, 1 ml M CaCl2, 1 ml M MgSO4 and 25 ml KPO4-buffer), and inoculated with E. coli OP50, and transferred every 3 days.
Strongyloides ratti L3 were obtained from the feces of infected rats following standardized procedures based on the Baermann technique at the Swiss Tropical and Public Health Institute. Freshly harvested S. ratti L3 were washed 3 times with PBS buffer and used immediately for in vitro drug testing. The species of nematodes used in the in vitro drug test was confirmed by a PCR targeting the 18S rRNA region of C. elegans, C. briggsae, C. remanei, C. brenneri, C. japonica and P. pacificus. Using the forward primer SSU18A and the reverse primer SSU26R [29] (Supplementary Table S1), a ∼950 bp fragment was amplified using FastStart High Fidelity PCR system (Roche). The reaction conditions were: 95°C for 10 min without Taq polymerase; 95°C for 2 min; 35 cycles of [95°C for 30 sec; 52°C for 30 sec; 72°C for 1 min 10 sec]; 72°C for 10 min. PCR products were purified using the Wizard SV PCR Clean-Up kit (Promega) and sequenced in both directions with SSU18A and SSU26R at Microsynth AG (http://www.microsynth.ch). Sequence quality check and assembly was done using 4Peaks (by A. Griekspoor and T. Groothuis; http://mekentosj.com) and a nucleotide blast was made on-line (NCBI) against the nucleotide collection (nt).
Drug test
Ivermectin and AAD-1566 were provided by Novartis Animal Health, Centre de Recherche Santé Animale, Fribourg, Switzerland. The compounds were diluted in pure DMSO to 10 mM and 250 mM, respectively. Appropriate dilutions of drugs were placed at the bottom of wells in 24-well plates and 1 ml NGM was added per well. The first well in each row served as a control with 1% DMSO. The plates were well shaken, allowed to dry at RT for several days, then inoculated with 10 µL E. coli OP50 and incubated at 37°C overnight. Eggs were purified from adults of the different species as follows: plates were washed with 3.5 ml water and incubated with 1.5 ml 5% bleach mixed 1∶1 with 5M NaOH for 10 min at room temperature. The eggs were washed with water and counted. A volume corresponding to 100–200 eggs, depending on the species, was added to each well of the drug plates. Plates were incubated at 20°C and scored microscopically for adults after 3 days and inspected again after 6 days for potential F2 generation of L1-larvae.
For the S. ratti in vitro tests 25 L3 larvae were incubated in 96-well plates containing 30 µl PBS buffer supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen) and appropriate drug dilutions. Control wells contained the highest percentage of solvent (2% DMSO). At each examination point (24, 48 and 72 h post-incubation) 15–20 µl of hot water (80°C) was added to each well, the larval movement observed and live worms counted using a dissecting microscope. All water stimulated wells were excluded from further reading. Half maximal effective concentration (EC50) values were calculated by non-linear regression of the sensitivity data, expressed as the percentage of surviving worms/larvae compared to the untreated control, to a sigmoidal dose-response curve of variable slope using Prism (GraphPad Prism version 5.00 for Mac OS X, GraphPad Software, San Diego California USA, http://www.graphpad.com).
Discovery of new Haemonchus contortus DEG-3 subfamily members
A previously described procedure was followed for RNA extraction, cDNA synthesis and rapid amplification of cDNA ends by PCR (RACE-PCR) for H. contortus [10]. Briefly, total RNA was extracted from a pool of approximately 50 adult nematodes. To generate cDNA, 1 µg of total RNA was reverse transcribed to cDNA using a d(T)30 primer and a SuperScript II Reverse Transcriptase (Invitrogen). For RACE-PCR, an internal reverse primer (Supplementary Table S1) was combined with splice leader sequence (1 or 2) to obtain the 5′ untranslated region (UTR), or an internal forward primer combined with a poly-dT primer for the 3′ UTR of the transcript. We cloned and sequenced the full-length Hco-acr-5, Hco-acr-17 and Hco-acr-24 coding sequence from H. contortus cDNA (GenBank accessions GU109271-GU109279) using primer pairs NheI_acr-5_frw2.1 and NotI_acr-5_rev2.1, NheI_acr-24_frw1 and XhoI_acr-24_rev1, NheI_acr-17_frw1 and XhoI_acr-17_rev1 (Supplementary Table S1). PCR products were gel purified using the Wizard SV PCR Clean-Up kit (Promega) and cloned into pCRII-TOPO (Invitrogen). Plasmid DNA was purified using the QIAprep Spin Miniprep kit (Qiagen) and three clones of each gene were sequenced using the standard primers M13 forward and reverse. The reported sequences in Supplementary Figures S1, S2 and S3 are each from one of the nearly identical single clones.
Accession numbers
Hco-acr-5, Hco-acr-17 and Hco-acr-24 coding sequence from H. contortus cDNA have been deposited with GenBank accessions GU109271-GU109279. MPTL-1 ACO48330 (GenBank).
SwissProt entries for mentioned proteins: ACC-1 Q21005_CAEEL, ACR-16 ACH1_CAEEL, ACR-17 P91320_CAEEL, ACR-20 B1Q281_CAEEL, ACR-21 Q9N5U8_CAEEL, ACR-23 O61884_CAEEL, ACR-5 ACR5_CAEEL, ACR-8 Q23355_CAEEL, AVR-14 Q95Q96_CAEEL, AVR-15 Q95PJ6_CAEEL, CUP-4 CUP4_CAEEL, DEG-3 ACH3_CAEEL, DES-2 ACH4_CAEEL, EXP-1 Q9TZI5_CAEEL, GAB-1 GBRB_CAEEL, GGR-2 Q2WF64_CAEEL, GLC-1 O17793_CAEEL, LEV-1 ACH7_CAEEL, LEV-8 Q93329_CAEEL, MOD-1 Q58AT9_CAEEL, PBO-5 Q67X94_CAEEL, UNC-29 ACH2_CAEEL, UNC-38 ACH5_CAEEL, UNC-49 Q0PDK2_CAEEL, UNC-63 ACH6_CAEEL, ZACN ZACN_HUMAN.
Results/Discussion
A phylogenomic pipeline
Caenorhabditis elegans peptide sequences annotated with the Gene Ontology term GO:0005230, ‘extracellular ligand-gated ion channel activity’, were retrieved from WormBase. These together with a similarly extracted set of human genes from uniprot and six H. contortus LGICs of the DEG-3 subfamily ([10] and Supplementary Figure S1, S2 and S3) were used as seed sequences for a Blast search against contiguous sequences from the abundant nematode, vertebrate and insect genome projects (Table 1). Caenorhabditis elegans is arguably the only finished eukaryote genome, but the genomes published as drafts are essentially complete, and several of the ongoing projects are well underway in terms of sequencing and assembly, only so far lacking in gene annotation. In this survey, we included data from 10 more nematode genomes, ranging from early shotgun stages to mature assemblies in annotation. Gene finding and annotation has become a major bottleneck, after next generation sequencing techniques accelerated sequence generation. By using Genewise to search the genome sequences directly we could also make use of unannotated genes. To assess nematode specificity of the herein predicted LGIC genes and to obtain more phylogenetic information, we also included three platyhelminth projects, four insect and nine vertebrate genomes (Table 1). No LGIC_LBD (from ligand binding domain, LBD) was found in eight plant species searched (www.gramene.org), which is in agreement with previous efforts [30]. The closest LGIC relatives in plants are highly diverged glutamate receptors [31]. Many plant toxins act on animal LGICs (e.g. curare, extracted from the plant Strychnos toxifera [32]). Due to the lack of LGICs, the toxic compounds pose little risk to the plants themselves.
Method recall ability
The automated approach identified 84 out of 102 annotated C. elegans LGICs using the LGIC_LBD profile alone. Only one additional LGIC was identified when the 39 membrane binding domain hits, from the LGIC_MEMB profile, were also included. The recall of the profile itself from full-length peptides was nearly complete. Using hmmer2, all 102 were found with the LBD profile and 98 with the MEMB profile. The lower complexity of the trans-membrane domains and a presumed lower need for conserved sequence specificity, together with the often extensive and variable internal loop between trans-membrane domain 3 (TMD3) and TMD4, all complicated by a slightly larger number of introns, apparently makes the LGIC_MEMB Hidden Markov Model profile less successful for finding family members directly from genomic nucleotide data.
A domain centric approach, as used here, is highly useful to compare the whole spectrum of LGICs. The domain approach is straightforward, can be applied directly on sequence data without prior exon prediction and gives alignments where the aligned positions are largely comparable. It would also be much more challenging to align the protein family meaningfully over the full length. Inclusion of more variable regions e.g. the internal loop between TMD3 and TMD4 would make the interpretation more difficult. While the recall of C. elegans receptor subunit genes by the identification of the LBD domain directly from the genome is not complete (80%), it is reassuring that the full-length peptide results for genomes, where such are available, are similar to the ones obtained through searches on the genomic DNA level, in particular in the DEG-3 subfamily.
Effects of genome project completeness
If genome sequence coverage is lacking altogether or if other problems keep the assembled contig size small, the number of LGICs predicted from our pipeline will be low. If the contigs with LGIC genes are too short so that they do not encompass the introns and exons for the LBD, they will not be detected by Genewise with a global (ls) type PFAM LGIC_LBD motif, even if fragments were detected by the initial BLAST screening.
To help assess the reliability of the number of genes found in the face of incompleteness of the ongoing projects, we measured the average contiguous sequence length (Table 1). Such a central measure can however be somewhat misleading for mature projects with a very high contig size variance. Indeed, the B. malayi and L. scapularis genome sequences show low sequence unit average length (<5 kbp), although the longest few contiguous sequences have considerable size (≫100 kbp; marked in Table 1). Gene counts for the genomes with average contig sizes below 3 kbp (Ascaris suum, Nippostrongylus brasiliensis and Teladorsagia circumcincta) in particular should not be taken for final.
Additional DEG-3 homologs
The full length coding sequences of H. contortus genes Hc-acr-17 (1590 bp), Hc-acr-5 (1833 bp) and Hc-acr-24 (1698 bp) were cloned by RACE PCR (see Materials and methods) and sequenced (Supplementary Figures S1, S2 and S3), helping to complete the understanding of individual DEG-3 subfamily members roles in monepantel drug action (Figure 2 and Table 2). Both Hco-acr-5 and Hco-acr-24 carried a spliced leader 2 (SL2) sequence at their 5′ end while Hco-acr-17 had a spliced leader 1 (SL1) sequence. The predicted LGIC proteins possess motifs typical for Cys-loop ligand-gated ion channels, including an N-terminal signal peptide, with the exception of Hco-acr-24 (as determined with Phobius [33], [34]), four transmembrane domains and the Cys-loop (two cysteines separated by 13 amino acids). Loops A to F, which are involved in ligand binding [35] are also present in the proteins. These loops are not annotated for Hco-acr-17 as the alignment with other related nAChRs were the loops location are known is poor. In loop C, there are two adjacent cysteines, defining Hco-acr-5, Hco-acr-17 and Hco-acr-24 as nAChR α subunits. Hco-acr-5 and Hco-acr-24 have the characteristic FxCC pattern, conserved among other ACR-5 and ACR-24 homologs, in contrast to Hco-acr-17 bearing the most common YxCC α subunit signature in loop C.
Fig. 2. Detailed view of the DEG-3 sub-family. 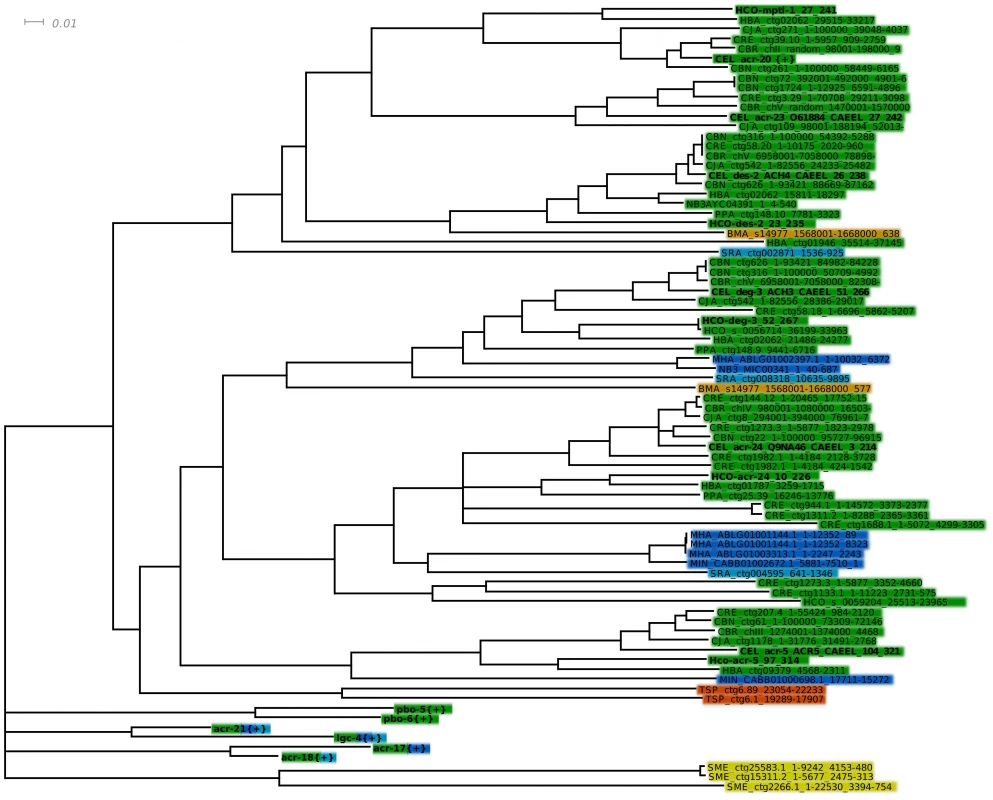
Detailed view of the DEG-3 sub-family from the LBD region NJ tree, with branches below 50% support after 1000 bootstrap iterations joined. A few related subfamilies are shown as collapsed branches. CEL, Caenorhabditis elegans, CBN, C. brenneri, CBR, C. briggsae, CRE, C. remanei, CJA, C. japonica, PPA, Pristionchus pacificus, HCO, Haemonchus contortus, HBA, Heterorhabditis bacteriophora, MHA, Meloidogyne hapla, NM3MIC/MIN, M. incognitia, NB3AYC Ancylostoma ceylanicum, SRA, Strongyloides ratti, BMA, Brugia malayi, TSP, Trichinella spiralis, SME, Schmidtea mediterranea. Pristionchus pacificus lacks a close MPTL-1 homolog and was predicted to be less sensitive to AAD-1566 than species such as C. japonica, H. contortus and H. bacteriophora. Tab. 2. Presence (+) or absence (−) of C. elegans homologous proteins of the DEG-3 subfamily members in various species. 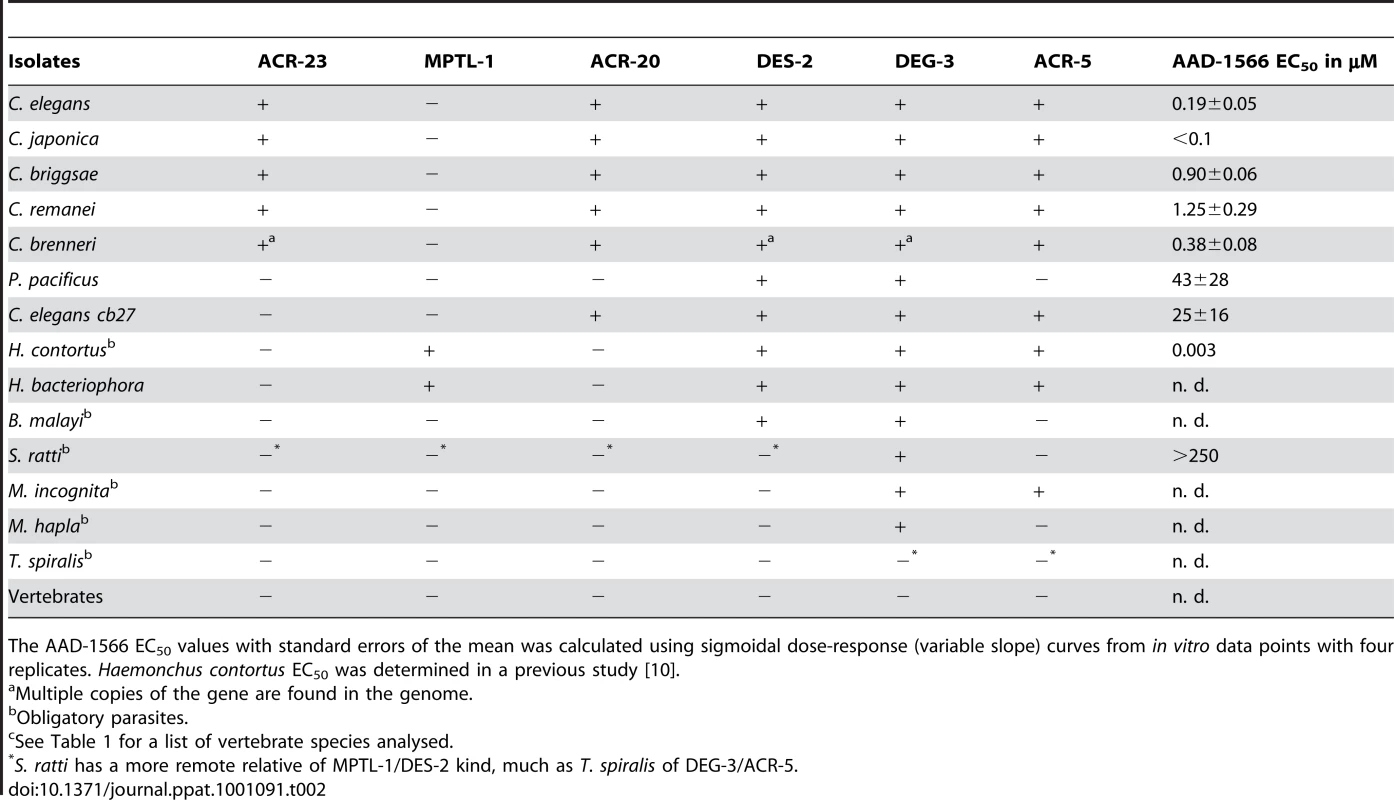
The AAD-1566 EC50 values with standard errors of the mean was calculated using sigmoidal dose-response (variable slope) curves from in vitro data points with four replicates. Haemonchus contortus EC50 was determined in a previous study [10]. Detecting LGICs
We used our phylogenomic pipeline on 33 genomes of varying levels of completeness, detecting 1273 putative genes bearing the PFAM LGIC_LBD motif (Table 1). The average number found in nematode genomes with an average sequence unit larger than 3 kbp was 56.1, whereas the same number was 41.7 for vertebrates, 18.0 for insects and 0 for plants. We also searched the nembase3 and nematode.org expressed sequence tag sets, finding a total of 27 LGICs with the LBD motif. An average of 31 and 57 LGICs were found in parasitic and non-parasitic organisms, respectively. The trend among the nematodes is clearly in agreement with the hypothesis that parasites have a reduced number of LGICs. It has been suggested that this could be a consequence of the less variable environment they encounter in comparison with their free-living relatives [19].
There is also considerable variation in LGIC number among the vertebrates (Table 1). The teleost genomes show a larger set of LGIC, in comparison to, for example, Bos taurus and Homo sapiens. The teleost repertoire appears to consist largely of multiple closely related variants of the terrestrial vertebrate LGIC types. The nematodes show a larger repertoire (Figure 1).
While the platyhelminthes included in the survey showed a smaller overall number of LGICs, they did have several unique types. LGIC subunits that are known to constitute part of drug target receptors are labelled in Figure 1. It is interesting to see how these drug target subunit genes form rather broad, i.e. member rich, yet nematode specific sub-branches of the superfamily tree. Importantly, the DEG-3 family appears nematode specific. In an optimistic outlook, several other such broad nematode specific branches exist in the tree, which could potentially be exploited as new anthelmintic targets.
Co-segregation with known named seed sequences in the bootstrapped tree was used for assigning putative identity to homologous genes. Interestingly, we found that neither P. pacificus nor S. ratti carries an ortholog of Hco-mptl-1 (Figure 2). Based on a single drug target model we thus predict P. pacificus and S. ratti to be insensitive to monepantel. We proceeded to test this hypothesis in vitro.
In vitro assay
An in vitro assay was established. Nematodes of one species were grown on 24 well NGM plates where each four well column was treated with a different drug concentration. An equal amount of eggs was added to each well, and the nematodes were scored microscopically after 3 and 6 days. A final concentration of 1% DMSO was used in all wells for the drug tests, including the no-drug controls (Figure 3, Supplementary Figure S4). All species tested (C. elegans, C. japonica, C. briggsae, C. brenneri, C. remanei, P. pacificus) tolerated up to 1% DMSO (Supplementary Figure S4 and Supplementary Table S2). For S. ratti the highest percentage of solvent (2% DMSO) found in the plate was used in the control wells and well tolerated.
Fig. 3. Sensitivity to AAD-1566 determined in vitro. 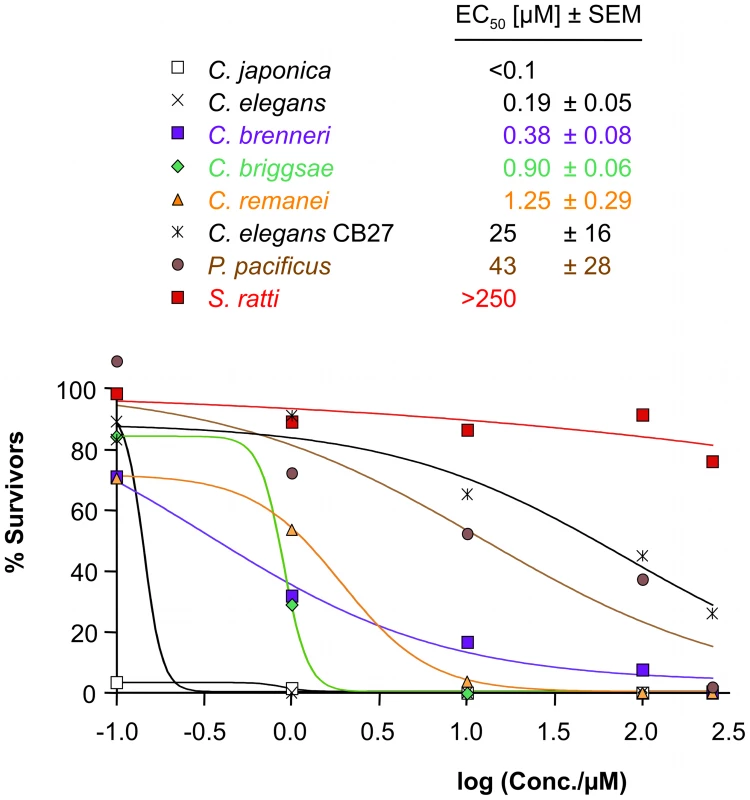
The percentage of the average number of adult worms present after 3 days exposure relative to a control is plotted versus drug concentration for Caenorhabditis elegans, mutated C. elegans strain acr-23 (cb27), C. japonica, C. briggsae, C. remanei, C. brenneri, Pristionchus pacificus and S. ratti. Sigmoid dose-response curve fit was performed in Prism. EC50 values with standard errors, estimated from data points with four replicates, are shown inset. All Clade V species tested (C. elegans, C. japonica, C. briggsae, C. brenneri, C. remanei, P. pacificus) exhibited a similar sensitivity to ivermectin (EC50≥10 nM; Supplementary Figure S7 and Supplementary Table S3). S. ratti L3 exposed to ivermectin at concentrations of 10 µM and above showed decreased survival rates 24–72 h post-incubation (EC50 of 13.6 µM 72 h post-incubation) (Supplementary Figure S7). This served as an additional positive control for the methods. It appears likely that drug sensitivity can be consistently determined for all strains. In a similar experiment the assayed nematode species showed varying degrees of concentration-dependent sensitivity towards DMSO, used as a solvent for the drugs. Even a compound with a small effect in this in vitro test could still be of therapeutic value, as levamisole clearly demonstrates. Levamisole does not directly kill the parasitic nematodes but creates a short term reversible paralysis, sufficient to allow the host to e.g. expulse the worms [12]. Up to mmolar concentrations of levamisole did not produce any effect detectable by our test readout (data not shown).
Hco-mptl-1 ortholog detection predicts AAD-1566 sensitivity - an MPTL-1/ACR-23 ortholog is the primary target
The present study tests the hypothesis that MPTL-1 is a major target of monepantel, since a nematocide effect on P. pacificus or S. ratti, which lacks an MPTL-1 homolog, would negate this. The P. pacificus genome has been published in a draft state, and as the assembly is nearly complete it is unlikely, but not impossible, that an eventual acr-23 ortholog could have been missed. The S. ratti genome is still in progress, but we were able to detect a subunit that appears branched prior to the ACR-23/MPTL-1/ACR-20 and the DES-2 split (see Figure 2 and Table 2). This species arguably helps us narrow down how deep the sensitivity to monepantel reaches in the tree.
Among the species C. elegans, C. japonica, C. briggsae, C. brenneri, C. remanei, P. pacificus as well as the mutated C. elegans strain acr-23 (cb27), C. japonica was the most sensitive to monepantel with EC50 values in the low nM range (Figure 3 and Table 1). This is comparable to the results previously obtained for H. contortus [10]. C. elegans was strongly affected at 1 µM, with an estimated EC50 of 0.19 µM. C. remanei, C. briggsae and C. brenneri showed similar EC50 values, but we found a comparatively large number of adult C. brenneri even at higher µM concentrations, e.g. 7.6% at 100 µM (Supplementary Table S4). C. brenneri has the largest assembly of LGICs in the study, and also possesses an extra DES-2 paralog and an additional ACR-23. Closer examination of the sequences of these copies did not present a convincing explanation of the diminished phenotype. One explanation may possibly lie in the gene doses of the channel subunits, leading to different stochiometries of the assembled channels, as has been observed in vitro [36].
The difference in EC50 value between C. japonica and to the other sensitive worms in the Caenorhabditis genera is already large. While we would not venture a molecular correlate, it is interesting to observe that the both the Cjp-ACR-23 and Cjp-ACR-20 seem to have diverged somewhat from the other sensitive Caenorhabditis worms, branching prior to them, possibly retaining more of an element important for high sensitivity, common with the earlier branched Hco-MPTL-1.
Pristionchus pacificus is rather insensitive to monepantel with an EC50 of 43 µM (Table 2, Figure 3). Furthermore, our in vitro test with S. ratti, bearing an early branching relative of ACR-20/ACR-23/MPTL-1, showed that monepantel lacks activity against S. ratti. A survival rate of 69% was observed after 72 h even with the highest concentration (250 µM) tested (Figure 3). A direct molecular mechanism is beyond the scope of the present investigation. However, we found that the phylogenomic detection of the ortholog of Hco-mptl-1, previously found mutated in strains insensitive to AAD-1566 [10], coincides with sensitivity to AAD-1566. This in agreement with our hypothesis that MPTL-1 is a major target of the drug.
Caenorhabditis elegans mutated in the DEG-3 family
acr-23 (cb27), a strain of C. elegans exhibiting a large deletion in Cel-acr-23 [8], was much less sensitive than wild type (genome strain N2), with an EC50 of 25 µM (Table 2, Figure 3). The difference in growth was marked and clearly visible to the naked eye (Supplementary Figure S5). This test can naturally not rule out the involvement of other LGIC subunits or indeed other genes in the susceptibility to AAD-1566. However, a set of C. elegans strains mutant only in other genes of the DEG-3 family (DEG-3/DES-2, ACR-5, ACR-18, and ACR-20) showed no loss of sensitivity towards AAD-1566 (Supplementary Figure S6 and Supplementary Table S5). This further strengthens the hypothesis that a subunit orthologous to MPTL-1/ACR-23 is required for the observed effect.
Detailed phenotype suggests additional secondary target – sensitivity stays in the family
For species that possess an MPTL-1 ortholog (e.g. C. elegans with Cel-ACR-23), AAD-1566 is lethal in vitro at nM concentrations, and a concentration-dependent retardation of development was observed. The strains without an Hco-MPTL-1 ortholog (P. pacificus and C. elegans acr-23 (cb27)) also exhibited a drug concentration-dependent developmental retardation. However, the substance was not lethal to them at the tested concentrations, as growth could still be observed after 6 days. Also in the case of S. ratti the survival rate of the larvae was slightly affected at high drug concentration (69% at 250 µM) and less at lower concentration. This suggests that there is at least one additional target.
One candidate is DES-2. In nematode strains selected for loss of sensitivity to AAD-1566, mutations in addition to those affecting Hco-mptl-1 were found in the Hco-des-2 gene 5′ UTR, introducing two novel upstream open reading frames, possibly reducing protein expression [10]. All tested species possess the DES-2 ortholog that bears the highest similarity to the established target outside the Cel-ACR-20/Cel-ACR-23 branch. If MPTL-1 is a primary target, causing high nematode lethality from AAD-1566, strains with modulations in the expression of a second target, DES-2, would only be selected for once MPTL-1 sensitivity was lost. It was noted in proof that in a recent study [37] Rufener et al. have expressed a functional H. contortus DES-2/DEG-3 channel in Xenopus oocytes that shows monepantel sensitivity. Though active against a range of clade V gastrointestinal nematodes, monepantel was reported to have only limited efficacy against Trichuris ovis (clade I) [38]. Genomic information to correlate this result with the absence of MPTL-1/ACR-23/DES-2 homologous subunits would be interesting.
There are a number of nematodes that, based on their complement of predicted nAChR genes, would be interesting to test for their sensitivity to AADs, but this would require other test methods. Two Meloidogyne species bear no close MPTL-1 homologs but have an ACR-5, homolog, which P. pacificus lacks. Heterorhabditis bacteriophora carries a DEG-3 family complement, which is highly similar to H. contortus, and we would thus predict a similar drug effect. Some important human parasitic nematodes of the clade I (Trichinella spiralis) and III (Brugia malayi) have more distant DES-2/DEG-3 homologs, much like Schmidtea mediteranea. A conjecture would be that they would show sensitivity only at a higher concentration. Tests on them could perhaps show what level of sequence identity is required, or what regions of the subunit need to be conserved, for any paralysis effect to be seen.
Conclusions
The family of LGIC provides many important drug and toxin targets, with nematodes bearing several unique subfamilies well diverged from those of other eukaryotes. We have constructed a simple phylogenomic pipeline to detect LGIC subunit genes. We survey the gene family in the many complete and ongoing sequencing projects in the nematode phylum and contrast these to genomes from some other relevant phyla to establish that the DEG-3 family indeed appears nematode specific to date. The survey also establishes the detection of drug sensitivity groups.
Given the hypothesis that an MPTL-1 homolog is the primary target of monepantel, the phylogenomic information gathered predicts P. pacificus and S. ratti to be insensitive to the drug, while four other model nematode species were predicted to be sensitive. These conjectures were tested experimentally. The in vitro effect of AAD-1566 on the panel of nematodes was found consistent with the hypothesis. All data point towards MPTL-1 as a primary target, in agreement with previous studies. We further hypothesise an additional secondary target for AAD-1566, possibly DES-2. This would explain a dose dependent growth retardation effect that is largely masked by the stronger, MPTL-1 mediated response.
Supporting Information
Zdroje
1. PerryBD
RandolphTF
1999 Improving the assessment of the economic impact of parasitic diseases and of their control in production animals. Vet Parasitol 84 145 168
2. DrudgeJH
LelandSEJr
WyantZN
1957 Strain variation in the response of sheep nematodes to the action of phenothiazine. II. Studies on pure infections of Haemonchus contortus. Am J Vet Res 18 317 325
3. AtanasioA
BoomkerJ
SitoeC
2002 A survey on the occurrence of resistance to anthelmintics of gastrointestinal nematodes of goats in Mozambique. Onderstepoort J Vet Res 69 215 220
4. ColesGC
RhodesAC
WolstenholmeAJ
2005 Rapid selection for ivermectin resistance in Haemonchus contortus. Vet Parasitol 129 345 347
5. JacksonF
CoopRL
2000 The development of anthelmintic resistance in sheep nematodes. Parasitology 120 Suppl S95 107
6. TraversaD
PaolettiB
OtrantoD
MillerJ
2007 First report of multiple drug resistance in trichostrongyles affecting sheep under field conditions in Italy. Parasitol Res 101 1713 1716
7. WaghornTS
LeathwickDM
RhodesAP
LawrenceKE
JacksonR
2006 Prevalence of anthelmintic resistance on sheep farms in New Zealand. N Z Vet J 54 271 277
8. KaminskyR
DucrayP
JungM
CloverR
RufenerL
2008 A new class of anthelmintics effective against drug-resistant nematodes. Nature 452 176 180
9. KaminskyR
GauvryN
Schorderet WeberS
SkripskyT
BouvierJ
2008 Identification of the amino-acetonitrile derivative monepantel (AAD 1566) as a new anthelmintic drug development candidate. Parasitol Res 103 931 939
10. RufenerL
MaserP
RoditiI
KaminskyR
2009 Haemonchus contortus acetylcholine receptors of the DEG-3 subfamily and their role in sensitivity to monepantel. PLoS Pathog 5 e1000380
11. MillarNS
GottiC
2009 Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 56 237 246
12. KohlerP
2001 The biochemical basis of anthelmintic action and resistance. Int J Parasitol 31 336 345
13. RobertsonAP
ClarkCL
BurnsTA
ThompsonDP
GearyTG
2002 Paraherquamide and 2-deoxy-paraherquamide distinguish cholinergic receptor subtypes in Ascaris muscle. J Pharmacol Exp Ther 302 853 860
14. Holden-DyeL
WalkerRJ
2006 Actions of glutamate and ivermectin on the pharyngeal muscle of Ascaridia galli: a comparative study with Caenorhabditis elegans. Int J Parasitol 36 395 402
15. ArenaJP
LiuKK
ParessPS
SchaefferJM
CullyDF
1992 Expression of a glutamate-activated chloride current in Xenopus oocytes injected with Caenorhabditis elegans RNA: evidence for modulation by avermectin. Brain Res Mol Brain Res 15 339 348
16. CulettoE
BaylisHA
RichmondJE
JonesAK
FlemingJT
2004 The Caenorhabditis elegans unc-63 gene encodes a levamisole-sensitive nicotinic acetylcholine receptor alpha subunit. J Biol Chem 279 42476 42483
17. FlemingJT
SquireMD
BarnesTM
TornoeC
MatsudaK
1997 Caenorhabditis elegans levamisole resistance genes lev-1, unc-29, and unc-38 encode functional nicotinic acetylcholine receptor subunits. J Neurosci 17 5843 5857
18. BrownLA
JonesAK
BuckinghamSD
MeeCJ
SattelleDB
2006 Contributions from Caenorhabditis elegans functional genetics to antiparasitic drug target identification and validation: nicotinic acetylcholine receptors, a case study. Int J Parasitol 36 617 624
19. WilliamsonSM
WalshTK
WolstenholmeAJ
2007 The cys-loop ligand-gated ion channel gene family of Brugia malayi and Trichinella spiralis: a comparison with Caenorhabditis elegans. Invert Neurosci 7 219 226
20. ChenN
HarrisTW
AntoshechkinI
BastianiC
BieriT
2005 WormBase: a comprehensive data resource for Caenorhabditis biology and genomics. Nucleic Acids Res 33 D383 389
21. The UniProt Consortium 2009 The Universal Protein Resource (UniProt). Nucleic Acids Res 37 D169 174
22. AltschulSF
GishW
MillerW
MyersEW
LipmanDJ
1990 Basic local alignment search tool. J Mol Biol 215 403 410
23. BirneyE
ClampM
DurbinR
2004 GeneWise and Genomewise. Genome Res 14 988 995
24. FinnRD
TateJ
MistryJ
CoggillPC
SammutSJ
2008 The Pfam protein families database. Nucleic Acids Res 36 D281 288
25. LarkinMA
BlackshieldsG
BrownNP
ChennaR
McGettiganPA
2007 Clustal W and Clustal X version 2.0. Bioinformatics 23 2947 2948
26. RiceP
LongdenI
BleasbyA
2000 EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16 276 277
27. HusonDH
RichterDC
RauschC
DezulianT
FranzM
2007 Dendroscope: An interactive viewer for large phylogenetic trees. BMC Bioinformatics 8 460
28. BinghamJ
SudarsanamS
2000 Visualizing large hierarchical clusters in hyperbolic space. Bioinformatics 16 660 661
29. BlaxterML
De LeyP
GareyJR
LiuLX
ScheldemanP
1998 A molecular evolutionary framework for the phylum Nematoda. Nature 392 71 75
30. TasneemA
IyerLM
JakobssonE
AravindL
2005 Identification of the prokaryotic ligand-gated ion channels and their implications for the mechanisms and origins of animal Cys-loop ion channels. Genome Biol 6 R4
31. ChiuJ
DeSalleR
LamHM
MeiselL
CoruzziG
1999 Molecular evolution of glutamate receptors: a primitive signaling mechanism that existed before plants and animals diverged. Mol Biol Evol 16 826 838
32. PhilippeG
AngenotL
TitsM
FrederichM
2004 About the toxicity of some Strychnos species and their alkaloids. Toxicon 44 405 416
33. KallL
KroghA
SonnhammerEL
2004 A combined transmembrane topology and signal peptide prediction method. J Mol Biol 338 1027 1036
34. KallL
KroghA
SonnhammerEL
2007 Advantages of combined transmembrane topology and signal peptide prediction–the Phobius web server. Nucleic Acids Res 35 W429 432
35. JonesAK
SattelleDB
2004 Functional genomics of the nicotinic acetylcholine receptor gene family of the nematode, Caenorhabditis elegans. Bioessays 26 39 49
36. WilliamsonSM
RobertsonAP
BrownL
WilliamsT
WoodsDJ
2009 The nicotinic acetylcholine receptors of the parasitic nematode Ascaris suum: formation of two distinct drug targets by varying the relative expression levels of two subunits. PLoS Pathog 5 e1000517
37. RufenerL
BaurR
KaminskyR
MaserP
SigelE
2010 Monepantel allosterically activates DEG-3/DES-2 channels of the gastrointestinal nematode Haemonchus contortus. Molecular Pharmacology In Press
38. SagerH
HoskingB
BapstB
SteinP
VanhoffK
2009 Efficacy of the amino-acetonitrile derivative, monepantel, against experimental and natural adult stage gastro-intestinal nematode infections in sheep. Vet Parasitol 159 49 54
39. GhedinE
WangS
SpiroD
CalerE
ZhaoQ
2007 Draft genome of the filarial nematode parasite Brugia malayi. Science 317 1756 1760
40. OppermanCH
BirdDM
WilliamsonVM
RokhsarDS
BurkeM
2008 Sequence and genetic map of Meloidogyne hapla: A compact nematode genome for plant parasitism. Proc Natl Acad Sci U S A 105 14802 14807
41. AbadP
GouzyJ
AuryJM
Castagnone-SerenoP
DanchinEG
2008 Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat Biotechnol 26 909 915
42. SteinLD
BaoZ
BlasiarD
BlumenthalT
BrentMR
2003 The genome sequence of Caenorhabditis briggsae: a platform for comparative genomics. PLoS Biol 1 E45
43. C. elegans Seq Consortium 1998 Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282 2012 2018
44. DieterichC
CliftonSW
SchusterLN
ChinwallaA
DelehauntyK
2008 The Pristionchus pacificus genome provides a unique perspective on nematode lifestyle and parasitism. Nat Genet 40 1193 1198
45. BerrimanM
HaasBJ
LoVerdePT
WilsonRA
DillonGP
2009 The genome of the blood fluke Schistosoma mansoni. Nature 460 352 358
46. AparicioS
ChapmanJ
StupkaE
PutnamN
ChiaJM
2002 Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science 297 1301 1310
47. Int. Chicken Genome Seq. Consortium 2004 Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 432 695 716
48. SheX
JiangZ
ClarkRA
LiuG
ChengZ
2004 Shotgun sequence assembly and recent segmental duplications within the human genome. Nature 431 927 930
49. ElsikCG
TellamRL
WorleyKC
GibbsRA
MuznyDM
2009 The genome sequence of taurine cattle: a window to ruminant biology and evolution. Science 324 522 528
50. Lindblad-TohK
WadeCM
MikkelsenTS
KarlssonEK
JaffeDB
2005 Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438 803 819
51. AdamsMD
CelnikerSE
HoltRA
EvansCA
GocayneJD
2000 The genome sequence of Drosophila melanogaster. Science 287 2185 2195
52. NeneV
WortmanJR
LawsonD
HaasB
KodiraC
2007 Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316 1718 1723
53. HoltRA
SubramanianGM
HalpernA
SuttonGG
CharlabR
2002 The genome sequence of the malaria mosquito Anopheles gambiae. Science 298 129 149
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent AutoimmunityČlánek Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on VirulenceČlánek Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral LoadČlánek A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 9- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Azole Drugs Are Imported By Facilitated Diffusion in and Other Pathogenic Fungi
- Two Genes on A/J Chromosome 18 Are Associated with Susceptibility to Infection by Combined Microarray and QTL Analyses
- Impact of Simian Immunodeficiency Virus Infection on Chimpanzee Population Dynamics
- Breaking the Stereotype: Virulence Factor–Mediated Protection of Host Cells in Bacterial Pathogenesis
- The Canine Papillomavirus and Gamma HPV E7 Proteins Use an Alternative Domain to Bind and Destabilize the Retinoblastoma Protein
- Rescue of HIV-1 Release by Targeting Widely Divergent NEDD4-Type Ubiquitin Ligases and Isolated Catalytic HECT Domains to Gag
- Steric Shielding of Surface Epitopes and Impaired Immune Recognition Induced by the Ebola Virus Glycoprotein
- Dynamics of the Multiplicity of Cellular Infection in a Plant Virus
- HLA Class I Binding of HBZ Determines Outcome in HTLV-1 Infection
- Pathogenic Bacteria Target NEDD8-Conjugated Cullins to Hijack Host-Cell Signaling Pathways
- The HA and NS Genes of Human H5N1 Influenza A Virus Contribute to High Virulence in Ferrets
- SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent Autoimmunity
- Cyclin-Dependent Kinase Activity Controls the Onset of the HCMV Lytic Cycle
- The N-Terminal Domain of the Arenavirus L Protein Is an RNA Endonuclease Essential in mRNA Transcription
- Generation of Neutralizing Antibodies and Divergence of SIVmac239 in Cynomolgus Macaques Following Short-Term Early Antiretroviral Therapy
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- Intracellular Proton Conductance of the Hepatitis C Virus p7 Protein and Its Contribution to Infectious Virus Production
- The Transcriptome of the Human Pathogen at Single-Nucleotide Resolution
- The Epidermal Growth Factor Receptor (EGFR) Promotes Uptake of Influenza A Viruses (IAV) into Host Cells
- Surface Co-Expression of Two Different PfEMP1 Antigens on Single -Infected Erythrocytes Facilitates Binding to ICAM1 and PECAM1
- Sequestration and Tissue Accumulation of Human Malaria Parasites: Can We Learn Anything from Rodent Models of Malaria?
- Phylogenomics of Ligand-Gated Ion Channels Predicts Monepantel Effect
- Generation of Covalently Closed Circular DNA of Hepatitis B Viruses via Intracellular Recycling Is Regulated in a Virus Specific Manner
- CpG-Methylation Regulates a Class of Epstein-Barr Virus Promoters
- Molecular and Evolutionary Bases of Within-Patient Genotypic and Phenotypic Diversity in Extraintestinal Infections
- A Bistable Switch and Anatomical Site Control Virulence Gene Expression in the Intestine
- Are Members of the Fungal Genus (a) Commensals; (b) Opportunists; (c) Pathogens; or (d) All of the Above?
- Structures of Receptor Complexes of a North American H7N2 Influenza Hemagglutinin with a Loop Deletion in the Receptor Binding Site
- Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral Load
- The Coevolution of Virulence: Tolerance in Perspective
- Involvement of the Cytokine MIF in the Snail Host Immune Response to the Parasite
- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
- High Content Phenotypic Cell-Based Visual Screen Identifies Acyltrehalose-Containing Glycolipids Involved in Phagosome Remodeling
- A Novel Small Molecule Inhibitor of Hepatitis C Virus Entry
- The Microbiota Mediates Pathogen Clearance from the Gut Lumen after Non-Typhoidal Diarrhea
- RNA Polymerases (L-Protein) Have an N-Terminal, Influenza-Like Endonuclease Domain, Essential for Viral Cap-Dependent Transcription
- Pathogen Specific, IRF3-Dependent Signaling and Innate Resistance to Human Kidney Infection
- Cellular Entry of Ebola Virus Involves Uptake by a Macropinocytosis-Like Mechanism and Subsequent Trafficking through Early and Late Endosomes
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Formation of Mobile Chromatin-Associated Nuclear Foci Containing HIV-1 Vpr and VPRBP Is Critical for the Induction of G2 Cell Cycle Arrest
- Association of Tat with Promoters of PTEN and PP2A Subunits Is Key to Transcriptional Activation of Apoptotic Pathways in HIV-Infected CD4+ T Cells
- Metal Hyperaccumulation Armors Plants against Disease
- Cyclin-Dependent Kinase-Like Function Is Shared by the Beta- and Gamma- Subset of the Conserved Herpesvirus Protein Kinases
- Role of Acetyl-Phosphate in Activation of the Rrp2-RpoN-RpoS Pathway in
- Ebolavirus Is Internalized into Host Cells Macropinocytosis in a Viral Glycoprotein-Dependent Manner
- A Novel Family of IMC Proteins Displays a Hierarchical Organization and Functions in Coordinating Parasite Division
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- The Coevolution of Virulence: Tolerance in Perspective
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



